Binary Molecular NomenclaturePage
1
1
Slide 1
Naming Binary Molecular Compounds
Slide 2
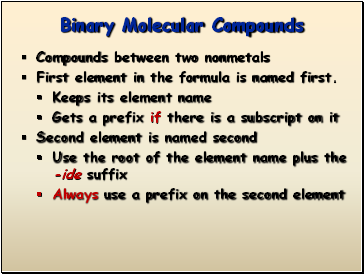
Binary Molecular Compounds
Compounds between two nonmetals
First element in the formula is named first.
Keeps its element name
Gets a prefix if there is a subscript on it
Second element is named second
Use the root of the element name plus the -ide suffix
Always use a prefix on the second element
Slide 3
List of Prefixes
1 = mon(o)
2 = di
3 = tri
4 = tetra
5 = penta
6 = hexa
7 = hepta
8 = octa
9 = nona
10 = deka
Slide 4
Naming Binary Compounds
P2O5 =
CO2 =
CO =
N2O =
diphosphorus pentoxide
carbon dioxide
carbon monoxide
dinitrogen monoxide
Slide 5
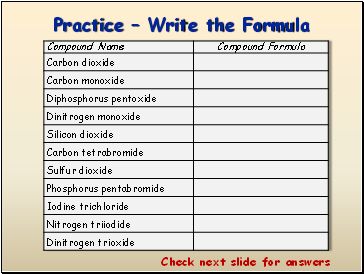
Practice – Write the Formula
Check next slide for answers
Slide 6
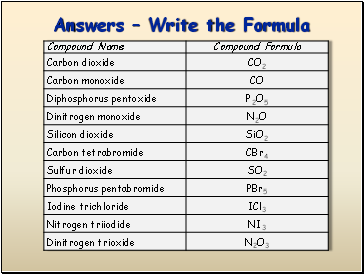
Answers – Write the Formula
Slide 7
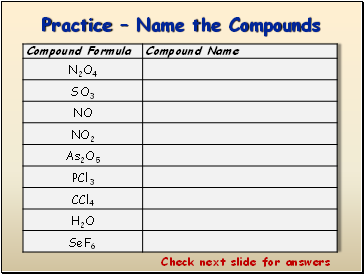
Practice – Name the Compounds
Check next slide for answers
Slide 8
Answers – Name the Compounds
Contents
Last added presentations
- Direct heat utilization of geothermal energy
- Health Physics
- Newton’s law of universal gravitation
- Newton’s third law of motion
- Sound
- Ch 9 Nuclear Radiation
- Friction
© 2010-2024 powerpoint presentations