Balancing Chemical Equations IPage
1
1
Slide 1
Balancing Chemical Equations
Just follow these steps…….
It’s quite easy really
Slide 2
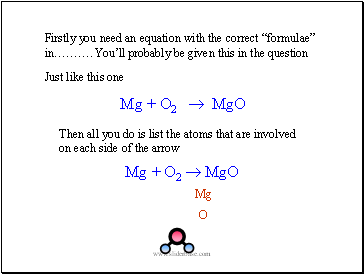
Firstly you need an equation with the correct “formulae” in………. You’ll probably be given this in the question
Just like this one
Mg + O2 MgO
Mg
O
Slide 3
[1] Just count up the atoms on each side
Then start balancing:
1
1
1
2
[2] The numbers aren’t balanced so then add “BIG” numbers to make up for any shortages
And adjust totals
2
2
2
Slide 4
But the numbers still aren’t equal, so add another “BIG” number
2
And adjust totals again
NOW BOTH SIDES HAVE EQUAL NUMBERS OF ATOMS
WE SAY THAT THE EQUATION IS BALANCED!!
2
Slide 5
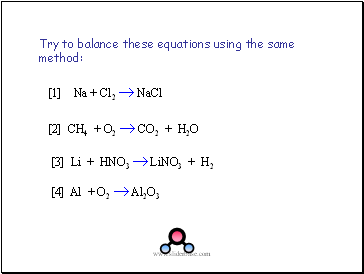
Try to balance these equations using the same method:
[1] Na + Cl2 NaCl
[2] CH4 + O2 CO2 + H2O
[4] Al + O2 Al2O3
[3] Li + HNO3 LiNO3 + H2
Slide 6
How did you get on??
[1] 2 Na + Cl2 2 NaCl
[2] CH4 + 2 O2 CO2 + 2 H2O
[4] 4 Al + 3 O2 2 Al2O3
[3] 2 Li + 2 HNO3 2 LiNO3 + H2
Here are the answers:
HOPE YOU’VE GOT THE IDEA… REMEMBER TO CHECK THAT YOU CAN DO ELECTROLYSIS EQUATIONS TOO
Slide 7
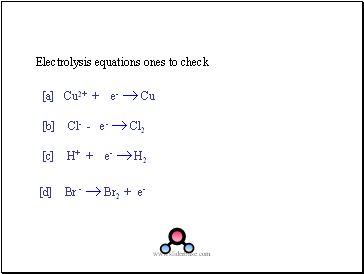
Electrolysis equations ones to check
[a] Cu2+ + e- Cu
[b] Cl- - e- Cl2
[c] H+ + e- H2
[d] Br - Br2 + e-
Slide 8
The answers are….
Remember to balance the charges on each side of the arrow
[a] Cu2+ + 2 e- Cu
[b] 2 Cl- - 2 e- Cl2
[c] 2 H+ + 2 e- H2
[d] 2 Br - Br2 + 2 e-
Slide 9
Hope that makes things a bit clearer….
Just remember it’s all about balance
Contents
Last added presentations
- Friction
- Direct heat utilization of geothermal energy
- Ch 9 Nuclear Radiation
- Waves & Sound
- Radiation Safety and Operations
- Upcoming Classes
- Newton’s third law of motion


![[1] Just count up the atoms on each side [1] Just count up the atoms on each side](images/referats/1121/image003.png)