Group 1Page
2
2
Recall how the alkali metals react with water and how this reaction changes as we travel down the group.
Recall whether these reactions are endothermic or exothermic.
Slide 9
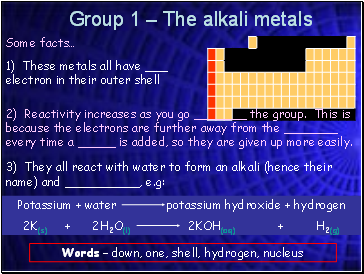
Group 1 – The alkali metals
1) These metals all have _ electron in their outer shell
Some facts…
2) Reactivity increases as you go _ the group. This is because the electrons are further away from the _ every time a _ is added, so they are given up more easily.
3) They all react with water to form an alkali (hence their name) and , e.g:
Words – down, one, shell, hydrogen, nucleus
Slide 10
Plenary 1.
Predict
What would happen if rubidium was added to water.
1. how fast will it react?
2. Will it be endothermic or exothermic?
3. What gas will be formed?
4. What would be the word equation
Slide 11
Plenary 2.
Predict
What would happen if caesium was added to water.
1. how fast will it react?
2. Will it be endothermic or exothermic?
3. What gas will be formed?
4. What would be the word equation?
1 2
Contents
Last added presentations
- Resource Acquisition and Transport in Vascular Plants
- Ch 9 Nuclear Radiation
- Waves & Sound
- Solar Thermal Energy
- Newton's laws of motion
- Friction
- Newton’s Laws of Motion