Group 1Page
1
1
Slide 1
Groups 1 - the Alkali Metals
Slide 2
Starter
What is the name of the group 1 elements?
Name 3 of these elements.
If one of these elements has the symbol and atomic number and mass
Na
What are the number of protons, neutrons and electrons in this atom?
What is the electron arrangement in this atom and how do we know what group we should place it in?
23
11
Slide 3
answers
What is the name of the group 1 elements?
Name 3 of these elements.
If one of these elements has the symbol and atomic number and mass
Na
What are the number of protons, neutrons and electrons in this atom?
What is the electron arrangement in this atom and how do we know what group we should place it in?
Alkali metals
Lithium, sodium, potassium, rubidium, caesium, francium
Protons = 11
Neutrons = 12
Electrons = 11
2,8,1
Placed in group 1 as the atom has one electron in its outer shell
23
11
Slide 4
Walt and WILF
WALT
Different elements have different properties related to their position in the Periodic Table
WILF
Locate the position in the Periodic Table of the alkali metals.
Recall how the alkali metals react with water and how this reaction changes as we travel down the group.
Recall whether these reactions are endothermic or exothermic.
Slide 5
Group 1 – The alkali metals
Slide 6
Groups 1 and 2
Groups 1 and 2 are always found in nature combined with other elements.
They’re called active metals because of their readiness to form new substances with other elements.
They are all metals except hydrogen, the first element in Group 1.
Representative Elements
Although hydrogen is placed in Group 1, it shares properties with the elements in Group 1 and Group 17.
Slide 7
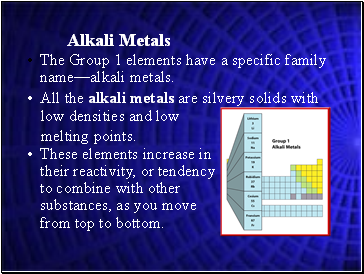
Alkali Metals
The Group 1 elements have a specific family name—alkali metals.
These elements increase in their reactivity, or tendency to combine with other substances, as you move from top to bottom.
Slide 8
Walt and WILF
WALT
Different elements have different properties related to their position in the Periodic Table
WILF
Locate the position in the Periodic Table of the alkali metals.
1 2
Contents
Last added presentations
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Friction
- History of Modern Astronomy
- Madame Marie Curie
- Newton’s Law of Gravity
- Mechanics Lecture
- Resource Acquisition and Transport in Vascular Plants