Periodic BehaviorPage
2
2
Definition: A measure of the ability of an atom in a chemical compound to attract electrons
Electronegativity tends to increase across a period
As radius decreases, electrons get closer to the bonding atomís nucleus
Electronegativity tends to decrease down a group or remain the same
As radius increases, electrons are farther from the bonding atomís nucleus
Slide 12
Periodic Table of Electronegativities
Slide 13
Periodic Trend: Electronegativity
Slide 14
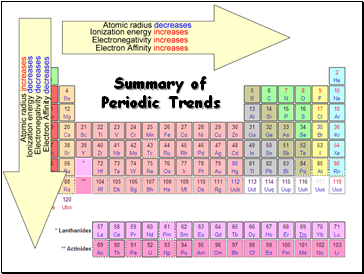
Summary of Periodic Trends
Slide 15
Ionic Radii
Cations
Positively charged ions formed when
an atom of a metal loses one or
more electrons
Smaller than the corresponding
atom
Anions
Negatively charged ions formed
when nonmetallic atoms gain one
or more electrons
Larger than the corresponding
atom
Slide 16
Table of Ion Sizes
Go to page:
1 2
1 2
Contents
- Atomic Radius
- Table of Atomic Radii
- Period Trend: Atomic Radius
- Ionization Energy
- Periodic Trend: Ionization Energy
- Electron Affinity
- Periodic Trend: Electron Affinity
- Electronegativity
- Periodic Table of Electronegativities
- Periodic Trend: Electronegativity
- Summary of Periodic Trends
- Ionic Radii
- Table of Ion Sizes
Last added presentations
© 2010-2025 powerpoint presentations