Lab ExperimentPage
1
1
Slide 1
DOUBLE REPLACEMENT
A LABORATORY EXAMINATION OF DOUBLE REPLACEMENT REACTION
Slide 2
SAFETY
GOGGLES
HAIR TIED BACK
APRONS
Slide 3
Chemical theory
THERE ARE MANY TYPES DOUBLE REPLACEMENT REACTIONS, SUCH AS ACID-BASE NEUTRALIZATION AND PRECIPITATION.
Slide 4
Precipitate reactions
THESE ARE REACTIONS IN WHICH AN INSOLUBLE SOLID IS FORMED AND SETTLES OUT OF SOLUTION, THE PRECIPITATE.
Slide 5
Example/form equation
AB + CD ----> AD + CB
THE CATIONS “TRADE” PLACES
Slide 6
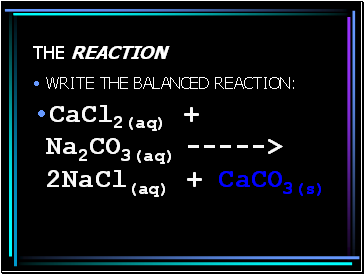
The reaction
WRITE THE BALANCED REACTION:
CaCl2(aq) + Na2CO3(aq) -----> 2NaCl(aq) + CaCO3(s)
Slide 7
Materials
RINGSTAND/WIRE TRIANGLE
FUNNEL
# 1 WHATMAN FILTER PAPER
Slide 8
MATERIALS
ASSORTED BEAKERS
ASSORTED FLASKS
GLASS STIRRING ROD
DRYING OVEN
Slide 9
Reagents
Na2CO3 SODIUM CARBONATE
CaCl2 CALCIUM CHLORIDE
Slide 10
Procedure
ADD 1 GRAM OF EACH “SALT” TO 20 mL OF WATER IN TWO SEPARATE BEAKERS AND DISSOLVE, THEN MIX SOLUTIONS IN COMMON BEAKER
Slide 11
Procedure continued
POUR THE CONTENTS OF THE BEAKER THROUGH THE FILTER PAPER FUNNEL ASSEMBLY
Slide 12
More procedure
REMEMBER, PRE-MASS THE DRY FILTER PAPER BEFORE FILTERING
Slide 13
Data and conclusions
CONSTRUCT A DATA TABLE
DESCRIBE WHY THE LAB RESULTS OCCURRED IN CONCLUSION
CALCULATE THEORETICAL YIELD, THEN PERCENT YIELD AND PERCENT ERROR
Slide 14
Technical writing portfolio
WRITING FOR A SPECIFIC PURPOSE
NO FLOWERY PROSE, GET TO THE POINT
MATH IS A LANGUAGE, CALCULATIONS ARE VITAL TO WRITEUP
Slide 15
1 2
Contents
- Chemical theory
- Precipitate reactions
- Example/form equation
- The reaction
- Materials
- Reagents
- Procedure
- Procedure continued
- More procedure
- Data and conclusions
- Technical writing portfolio
- Purpose
- Audience
- Organization
- Writing
- Components
- More components
- Still more components
- Nuts & bolts
Last added presentations
- Thermal Energy
- Ch 9 Nuclear Radiation
- Newton’s Law of Gravity
- Health Physics
- Heat-Energy on the Move
- Sound
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal