Balancing Chemical Equations. Balancing redox equationsPage
3
3
OIL RIG
Oxidation can be defined (in terms of electon transfer) as the loss of electrons from a species
Reduction can be defined as the gain of electrons by a species
An oxidising agent (or electron acceptor) must itself be reduced
A reducing agent (or electron donor) must itself be oxidised
The action of both oxidising and reducing agents can be represented in the form of a half-equation
Slide 11
Identifying Oxidising and Reducing Agents
Example
Formation of calcium oxide (CaO)
2Ca(s) + O2(g) 2CaO(s)
Ca and O2 (reactants): neutral atoms
Calcium oxide (product): ionic compound
Ca2+ O2- ions
Can write the reaction as two half-reactions:
2Ca 2Ca2+
O2 2O2-
Here, calcium is oxidised; i.e. it loses electrons to oxygen
It reduces the oxygen
it acts as a reducing agent
Oxygen is reduced; i.e. it gains electrons from calcium
It oxidises the calcium
it acts as an oxidising agent
+ 4e-
+ 4e-
OIL
RIG
Slide 12
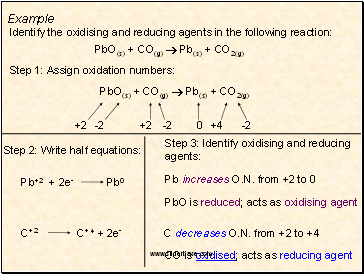
Example
PbO(s) + CO(g) Pb(s) + CO2(g)
Identify the oxidising and reducing agents in the following reaction:
+2
-2
+2
-2
0
+4
-2
Pb increases O.N. from +2 to 0
PbO is reduced; acts as oxidising agent
C decreases O.N. from +2 to +4
CO is oxidised; acts as reducing agent
Step 1: Assign oxidation numbers:
PbO(s) + CO(g) Pb(s) + CO2(g)
Step 2: Write half equations:
Step 3: Identify oxidising and reducing agents:
Slide 13
Questions
Identify the oxidising and reducing agents and write the half reactions in
the following:
(c) 4Fe +3O2 2Fe2O3
Answer: Fe Fe3+ + 3e-
O2 + 4e- 2O2-
O2 : oxidising agent Fe: reducing agent
(b) Si + 2F2 SiF4
Answer: Si Si4+ + 4e-
F2 + 2e- 2F-
F2 : oxidising agent Si : reducing agent
(a) Cl2 + 2NaBr 2NaCl + Br2
Answer: 2Br-- Br2 + 2e-
Cl2 + 2e- 2Cl-
Cl2 : oxidising agent Br - : reducing agent
Slide 14
Balancing redox equations
Example
Balance the equation showing the oxidation of Fe2+ ions to Fe3+ ions by dichromate ions (Cr2O72-) in an acidic medium
Fe2+ + Cr2O72- Fe3+ + Cr3+
Step 1: Identify oxidising and reducing agents and write half reactions
Fe2+ : +2
Cr2O72- : 2Cr + 7(-2) = -2
2Cr = 12
Cr = +6
Fe3+ : +3
Cr3+ : +3
Fe2+ Fe3+
Iron loses one electron, i.e. it is oxidised. It donates one electron to dichromate, i.e. it is the reducing agent.
Contents
- Balancing Chemical Equations
- Balancing redox reactions
- Rules for assigning oxidation numbers (O.N.)
- Defining Oxidising and Reducing agents
- Identifying Oxidising and Reducing Agents
- Balancing redox equations
Last added presentations
- Waves & Sound
- Geophysical Concepts, Applications and Limitations
- Newton’s laws of motion
- Radiation Safety and Operations
- Sensory and Motor Mechanisms
- Motion
- Newton’s Laws of Motion