Elements compounds and mixturesPage
4
4
Slide 21
Mixtures
A mixture of lead atoms and chlorine atoms. They exist in no particular ratio and are not chemically combined with each other. They can be separated by physical means.
A mixture of PbCl2 and PbCl4 formula units. Again, they are in no particular ratio to each other and can be separated without chemical change.
منگل، 18 ذو القعد، 1436
21
Slide 22
Characteristics of mixture
It is an impure substance
No formula
They can be mixed in any ratio.
The properties of the mixture are the properties of its constituents.
Constituents can be easily seperated by physical methods e.g. heating, drying, crystallization, distillation etc.
It is either homogenous or heterogenous.
22
منگل، 18 ذو القعد، 1436
Slide 23
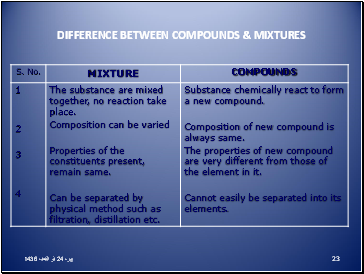
Difference between compounds & mixtures
23
منگل، 18 ذو القعد، 1436
Slide 24
24
منگل، 18 ذو القعد، 1436
Slide 25
Physical vs. Chemical Properties
Physical properties can be measure without changing the basic identity of the substance (e.g., color, density, odor, melting point)
Chemical properties describe how substances react or change to form different substances (e.g., hydrogen burns in oxygen)
Intensive physical properties do not depend on how much of the substance is present.
Examples: density, temperature, and melting point.
Extensive physical properties depend on the amount of substance present.
Examples: mass, volume, pressure.
25
منگل، 18 ذو القعد، 1436
Slide 26
Physical and Chemical Changes
When a substance undergoes a physical change, its physical appearance changes.
Ice melts: a solid is converted into a liquid.
Physical changes do not result in a change of composition.
When a substance changes its composition, it undergoes a chemical change:
When pure hydrogen and pure oxygen react completely, they form pure water. In the flask containing water, there is no oxygen or hydrogen left over.
26
منگل، 18 ذو القعد، 1436
Contents
- Classification of Matter
- Pure Substances and Mixtures
- Characteristics of pure & impure substances
- Elements
- Classification of Elements as Metals & Non- Metals
- Elements & symbols
- Compounds
- Characteristics of compound
- Compounds
- Types of Compounds
- Ionic Compounds
- Molecular Compounds
- Network Solids
- Examples of some formula
- Mixtures
- Characteristics of mixture
- Difference between compounds & mixtures
- Physical vs. Chemical Properties
- Physical and Chemical Changes
- Testing the purity of a substance
Last added presentations
- Newton’s law of universal gravitation
- Madame Marie Curie
- Sound
- Health Physics
- Resource Acquisition and Transport in Vascular Plants
- Motion
- Space Radiation