Elements compounds and mixturesPage
1
1
Slide 1
ELEMENTS, COMPOUNDS & MIXTURES
By Muhammad Ali
1
منگل، 18 ذو القعد، 1436
Slide 2
Classification of Matter
2
منگل، 18 ذو القعد، 1436
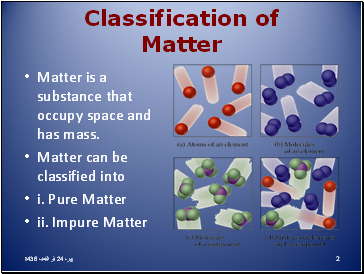
Matter is a substance that occupy space and has mass.
Matter can be classified into
i. Pure Matter
ii. Impure Matter
Slide 3
Pure Substances and Mixtures
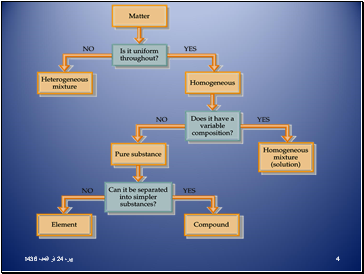
If matter is not uniform throughout, then it is a heterogeneous mixture.
If matter is uniform throughout, it is homogeneous.
If homogeneous matter can be separated by physical means, then the matter is a mixture.
If homogeneous matter cannot be separated by physical means, then the matter is a pure substance.
If a pure substance can be decomposed into something else, then the substance is a compound.
If a pure substance cannot be decomposed into something else, then the substance is an element.
3
منگل، 18 ذو القعد، 1436
Slide 4
4
منگل، 18 ذو القعد، 1436
Slide 5
Characteristics of pure & impure substances
A pure substance boils at a constant temperature i.e. it has a fix boiling point. An impure liquid could boil higher than the expected boiling point and over a range of temperature.
A pure substance melts quite sharply at the melting point. An impure solid melts below its expected melting point and more slowly over a wider temperature range.
5
منگل، 18 ذو القعد، 1436
Slide 6
Elements
Element consist of unique type of atoms.
Element cannot be further broken into simple substance by any chemical or physical means.
There are 118 elements known.
Each element is given a unique chemical symbol (one or two letters).
Elements are building blocks of matter.
6
منگل، 18 ذو القعد، 1436
Slide 7
Elements
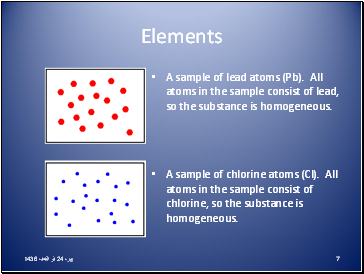
A sample of lead atoms (Pb). All atoms in the sample consist of lead, so the substance is homogeneous.
Contents
- Classification of Matter
- Pure Substances and Mixtures
- Characteristics of pure & impure substances
- Elements
- Classification of Elements as Metals & Non- Metals
- Elements & symbols
- Compounds
- Characteristics of compound
- Compounds
- Types of Compounds
- Ionic Compounds
- Molecular Compounds
- Network Solids
- Examples of some formula
- Mixtures
- Characteristics of mixture
- Difference between compounds & mixtures
- Physical vs. Chemical Properties
- Physical and Chemical Changes
- Testing the purity of a substance
Last added presentations
- Heat-Energy on the Move
- Upcoming Classes
- Geophysical Concepts, Applications and Limitations
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Newton’s law of universal gravitation
- Health Physics
- Simulation at NASA for the Space Radiation Effort