Earth HistoryPage
1
1
Slide 1
Atmospheric Chemistry
Formation of the Atmosphere
The Early Atmosphere
Origin of Life and Oxygen
Ozone
Air Pollution
Acid Rain
Greenhouse Effect
Slide 2
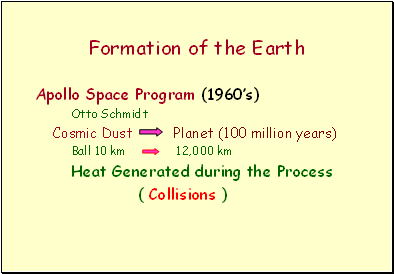
Formation of the Earth
Apollo Space Program (1960’s)
Otto Schmidt
Cosmic Dust Planet (100 million years)
Ball 10 km 12,000 km
Heat Generated during the Process
( Collisions )
Differentiation Occurs
Slide 3
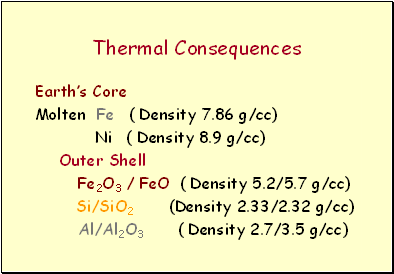
Thermal Consequences
Earth’s Core
Molten Fe ( Density 7.86 g/cc)
Ni ( Density 8.9 g/cc)
Outer Shell
Fe2O3 / FeO ( Density 5.2/5.7 g/cc)
Si/SiO2 (Density 2.33/2.32 g/cc)
Al/Al2O3 ( Density 2.7/3.5 g/cc)
Slide 4
Formation of the Mantle
The less dense material will go toward the surface (Polar Oxides of Si, Al, Fe)
Separation will occur as Fe/Ni core is nonpolar
MANTLE
starts to form and cool
(Production of Iron from Iron Ore)
Slide 5
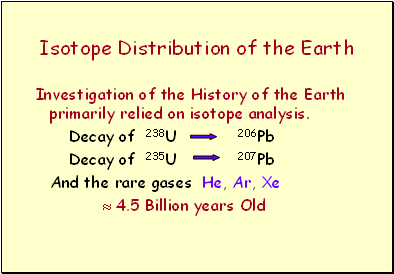
Isotope Distribution of the Earth
Investigation of the History of the Earth primarily relied on isotope analysis.
Decay of 238U 206Pb
Decay of 235U 207Pb
And the rare gases He, Ar, Xe
4.5 Billion years Old
Slide 6
Appearance of the Atmosphere
Did the atmosphere suddenly appear ?
Isotope Analysis gives a clue
Claude Allegre He, Ar & Xe
( Rare Gases do not react readily )
Argon has three isotopes
(36Ar 0.337) (38Ar 0.063) (40Ar 99.60) EC Decay 40K 40Ar
( t1/2 = 1.28 x 109y )
Slide 7
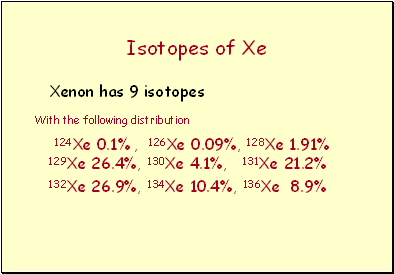
Isotopes of Xe
Xenon has 9 isotopes
With the following distribution
124Xe 0.1% , 126Xe 0.09%, 128Xe 1.91% 129Xe 26.4%, 130Xe 4.1%, 131Xe 21.2%
132Xe 26.9%, 134Xe 10.4%, 136Xe 8.9%
Slide 8
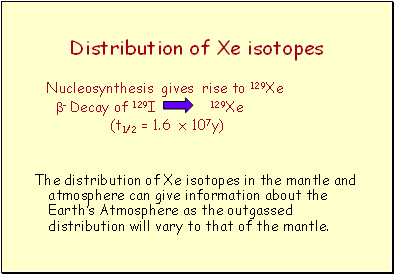
Distribution of Xe isotopes
Nucleosynthesis gives rise to 129Xe
- Decay of 129I 129Xe
(t1/2 = 1.6 x 107y)
The distribution of Xe isotopes in the mantle and atmosphere can give information about the Earth’s Atmosphere as the outgassed distribution will vary to that of the mantle.
Slide 9
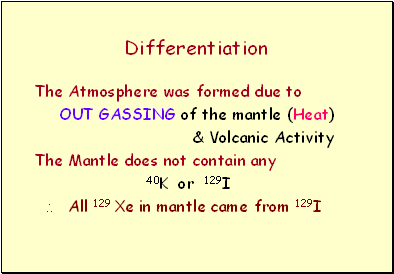
Differentiation
The Atmosphere was formed due to
OUT GASSING of the mantle (Heat)
& Volcanic Activity
Contents
- Atmospheric Chemistry
- Formation of the Earth
- Thermal Consequences
- Formation of the Mantle
- Isotope Distribution of the Earth
- Appearance of the Atmosphere
- Isotopes of Xe
- Distribution of Xe isotopes
- Differentiation
- Age of differentiation
- Ratios of Isotopes
- Conclusions from Isotope Analysis
- Collecting the evidence
- Early Atmosphere
- Origin of Life
- Formation of Simple Amino Acids
- Murchison Meteor
- Early Energy System
- Role of Blue Green Algae
- Decline of Anaerobic Bacteria
- Oxygen Rich Planet
- The trouble with oxygen
- The present atmosphere
- Distance from the Sun
- Influence of Earth’s Mass
- Escape Velocity
- No H or He in Earth’s Atmosphere
- Little CO2 in atmosphere
- Earth ,Venus & Mars
- Distribution of Gases on Earth Venus & Mars
- Role of Shellfish
- Triple point of H2O
- Water ( Solid,Liquid, Gas)
- Super Greenhouse & Acid Rain
- Current Atmosphere
- Present Level of Oxygen
- Structure of Atmosphere
- Ozone Layer
- Ozone and Radiation
- Effects of Reduction in Ozone
- Chlorofluorocarbons & Ozone
- Ozone Protection
- Ozone Destruction
- Control of CFC’s
- Uses of CFC’s
- Lifetime of CFC’s
- Naming of CFC’s
- Chloromonoxide
- Relationship between ClO. & O3
- Thickness of Ozone Layer
- Other Ozone Depleters
- Interactive Catalytic Forms
- Origin of Ozone Hole
- Ice crystal formation
- Possible Role of CO2
- Impenetrable Vortex formation
- PSC’s
- HCL attachment
- Role of ClONO2
- Formation of Cl. Radicals
- Hole Closure
- Dimer ClOOCl
- Antarctic and Arctic Vortexes
- Possible Link
- Further Reading
Last added presentations
- Newton’s law of universal gravitation
- Simulation at NASA for the Space Radiation Effort
- Thermal Energy
- Newton’s Laws of Motion
- History of Modern Astronomy
- Solar Energy
- Radiation