Chatelier's PrinciplePage
1
1
Slide 1
Le Chatelierís Principle
Slide 2
LeChatelierís Principle
When a system at
equilibrium is placed under
stress, the system will
undergo a change in such
a way as to relieve that
stress and restore a
state of equilibrium.
Henry Le Chatelier
Slide 3
Le Chatelier Translated
When you take something away from a system at equilibrium, the system shifts in such a way as to replace some what youíve taken away.
When you add something to a system at equilibrium, the system shifts in such a way as to use up some of what youíve added.
Slide 4
LeChatelier Example
A closed container of ice and water is at equilibrium. Then, the temperature is raised.
Ice + Energy Water
The system temporarily shifts to the _ to restore equilibrium.
right
Slide 5
LeChatelier Example #2
A closed container of N2O4 and NO2 is at equilibrium. NO2 is added to the container.
N2O4 (g) + Energy 2 NO2 (g)
The system temporarily shifts to the _ to restore equilibrium.
left
Slide 6
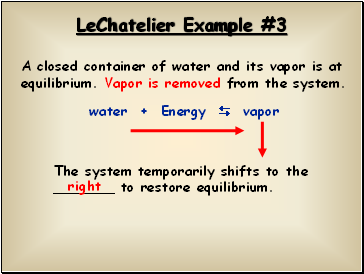
LeChatelier Example #3
A closed container of water and its vapor is at equilibrium. Vapor is removed from the system.
water + Energy vapor
The system temporarily shifts to the _ to restore equilibrium.
right
Slide 7
LeChatelier Example #4
A closed container of N2O4 and NO2 is at equilibrium. The pressure is increased.
N2O4 (g) + Energy 2 NO2 (g)
The system temporarily shifts to the _ to restore equilibrium, because there are fewer moles of gas on that side of the equation.
left
Contents
Last added presentations
- Motion
- Newtonís third law of motion
- Radioactivity and Nuclear Reactions
- Newtonís Law of Gravity
- Ch 9 Nuclear Radiation
- Friction
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal