Calculation of Solution ConcentrationPage
1
1
Slide 1
Solution Concentration
Slide 2
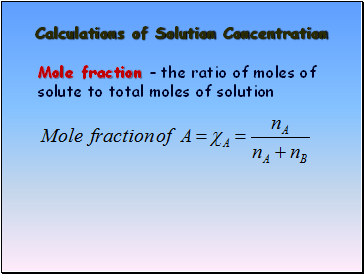
Calculations of Solution Concentration
Mole fraction – the ratio of moles of solute to total moles of solution
Slide 3
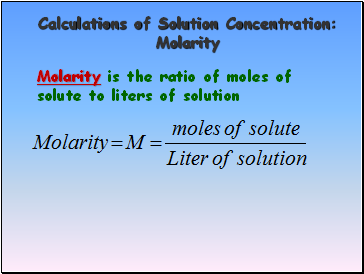
Calculations of Solution Concentration: Molarity
Molarity is the ratio of moles of solute to liters of solution
Slide 4
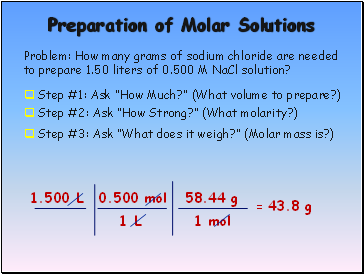
Preparation of Molar Solutions
Problem: How many grams of sodium chloride are needed to prepare 1.50 liters of 0.500 M NaCl solution?
Step #1: Ask “How Much?” (What volume to prepare?)
1.500 L
Step #2: Ask “How Strong?” (What molarity?)
0.500 mol
1 L
Step #3: Ask “What does it weigh?” (Molar mass is?)
58.44 g
1 mol
= 43.8 g
Slide 5
Serial Dilution
Problem: What volume of stock (11.6 M) hydrochloric acid is needed to prepare 250. mL of 3.0 M HCl solution?
MstockVstock = MdiluteVdilute
(11.6 M)(x Liters) = (3.0 M)(0.250 Liters)
x Liters = (3.0 M)(0.250 Liters)
11.6 M
= 0.065 L
Contents
Last added presentations
- Radioactivity and Nuclear Reactions
- Newton’s Law of Gravity
- Soil and Plant Nutrition
- History of Modern Astronomy
- Thermal Energy
- Space Radiation
- Health Physics