Basic ChemistryPage
1
1
Slide 1
Chemistry
Slide 2
Matter
Matter = any material substance with Mass & Volume
Slide 3
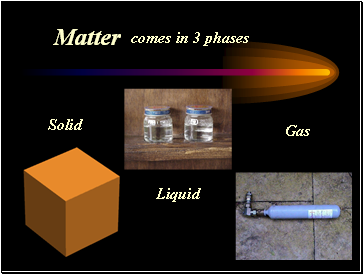
Matter
comes in 3 phases
Slide 4
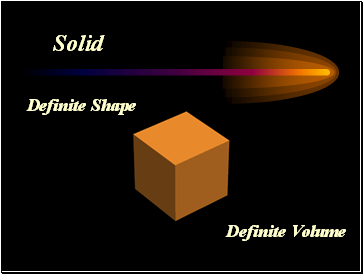
Solid
Definite Shape
Definite Volume
Slide 5
Liquid
Indefinite Shape – takes the shape of the container
Definite Volume
Slide 6
Gas
Indefinite Shape – takes the shape of the container
Indefinite Volume – can expand and be compressed
Slide 7
Elements
one of the 100+ pure substances
that make up everything in the universe
Slide 8
Examples of Elements
Slide 9
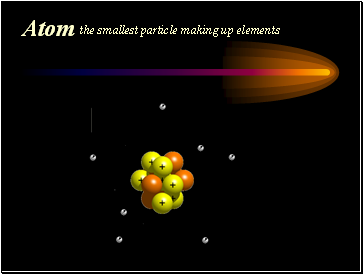
Atom
the smallest particle making up elements
Slide 10
Sub-atomic Particles
Protons p+ - positive charge, in nucleus
Electrons - e- negative charge, orbiting nucleus
Neutrons n0 – no charge, in nucleus
Slide 11
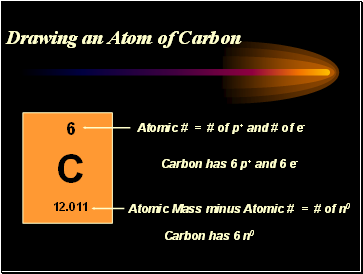
Drawing an Atom of Carbon
minus Atomic # = # of n0
= # of p+ and # of e-
Carbon has 6 p+ and 6 e-
Carbon has 6 n0
Slide 12
Drawing an Atom of Carbon
Slide 13
Compounds
Compounds - 2 or more elements chemically combined to form a new substance with new properties
Properties – The way a chemical substance looks and behaves
Slide 14
Compounds
Compounds – are made of 2 or more different atoms combined to form Molecules
Chemical formula lists the number of different atoms in a single molecule
Structural formula shows the arrangement of the atoms in a single molecule
Slide 15
Molecules
Glucose Sugar
Slide 16
Compounds
1 2
Contents
- Matter
- Solid
- Liquid
- Gas
- Elements
- Examples of Elements
- Atom
- Sub-atomic Particles
- Drawing an Atom of Carbon
- Compounds
- Molecules
- Examples of Inorganic Compounds
Last added presentations
- Health Physics
- Heat-Energy on the Move
- History of Modern Astronomy
- Newton’s law of universal gravitation
- Resource Acquisition and Transport in Vascular Plants
- Upcoming Classes
- Ch 9 Nuclear Radiation