Acid-Base ReactionsPage
1
1
Slide 1
Acids and Bases
Slide 2
Properties of Acids
Acids are proton (hydrogen ion, H+) donors
Acids have a pH lower than 7
Acids taste sour
Acids effect indicators
Blue litmus turns red
Methyl orange turns red
Acids react with active metals, producing H2
Acids react with carbonates
Acids neutralize bases
Slide 3
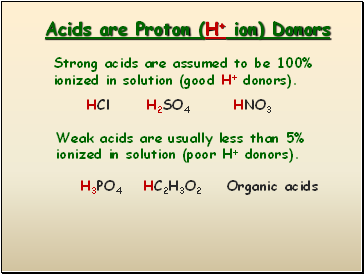
Acids are Proton (H+ ion) Donors
Strong acids are assumed to be 100% ionized in solution (good H+ donors).
Weak acids are usually less than 5% ionized in solution (poor H+ donors).
HCl
H2SO4
HNO3
H3PO4
HC2H3O2
Organic acids
Slide 4
Acids Have a pH less than 7
Slide 5
Acids Effect Indicators
Blue litmus paper turns red in contact with an acid.
Methyl orange turns red with addition of an acid
Slide 6
Acids React with Active Metals
Acids react with active metals to form salts and hydrogen gas.
Mg + 2HCl MgCl2 + H2(g)
Zn + 2HCl ZnCl2 + H2(g)
Mg + H2SO4 MgSO4 + H2(g)
Slide 7
Acids React with Carbonates
2HC2H3O2 + Na2CO3
2 NaC2H3O2 + H2O + CO2
Slide 8
Effects of Acid Rain on Marble (calcium carbonate)
George Washington:
BEFORE
George Washington:
AFTER
Slide 9
Acids Neutralize Bases
HCl + NaOH NaCl + H2O
Neutralization reactions ALWAYS produce a salt and water.
H2SO4 + 2NaOH Na2SO4 + 2H2O
2HNO3 + Mg(OH)2 Mg(NO3)2 + 2H2O
Slide 10
Properties of Bases
Bases are proton (hydrogen ion, H+) acceptors
Bases have a pH greater than 7
Bases taste bitter
Bases effect indicators
Red litmus turns blue
Phenolphthalein turns purple
Solutions of bases feel slippery
Bases neutralize acids
Slide 11
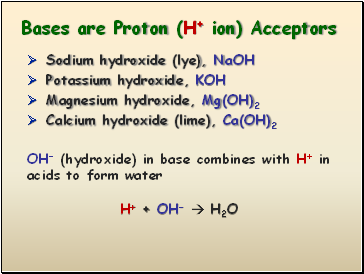
Bases are Proton (H+ ion) Acceptors
Sodium hydroxide (lye), NaOH
Potassium hydroxide, KOH
Magnesium hydroxide, Mg(OH)2
Calcium hydroxide (lime), Ca(OH)2
OH- (hydroxide) in base combines with H+ in acids to form water
1 2
Contents
- Acids and Bases
- Properties of Acids
- Acids are Proton (H+ ion) Donors
- Acids Effect Indicators
- Acids React with Active Metals
- Acids React with Carbonates
- Acids Neutralize Bases
- Properties of Bases
- Bases Effect Indicators
- Bases Neutralize Acids
Last added presentations
- Newton’s laws of motion
- Sound
- Ch 9 Nuclear Radiation
- Soil and Plant Nutrition
- The Effects of Radiation on Living Things
- Geophysical Concepts, Applications and Limitations
- Upcoming Classes