Why the sky changes colourPage
1
1
Slide 1
Why Does the Sky Change Colour as the Day Progresses?
Chitra Patil Mr. McClure SCH 4U0 18 December 2013
Slide 2
Every colour change in sky is related to light and atmosphere Colour is what the human eye perceives when light is given off
Slide 3
Light
Slide 4
Light
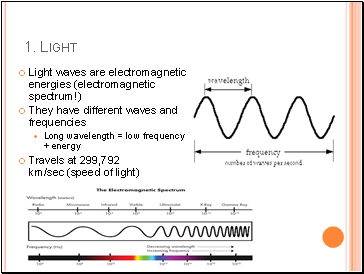
Light waves are electromagnetic energies (electromagnetic spectrum!)
They have different waves and frequencies
Long wavelength = low frequency + energy
Travels at 299,792 km/sec (speed of light)
Slide 5
1. Light (cont’d)
Visible light looks white:
White is made up of different colours (monochromatic light)
Each colour is related to size of wavelength, frequency, and energy
Sun’s colours are arranged from short to long
Light with shorter wavelengths (ie: blue) are more easily absorbed in sky
Rayleigh scattering: process of absorbing light and scatter particles
Slide 6
Atmosphere
Slide 7
Atmosphere
Molecules present:
Carbon dioxide
Ozone
Hydrogen gas
Nitrogen gas
Oxygen gas
When light is shone, N2 gas and O2 gas will absorb the most energy…
Let’s focus on these
Slide 8
Atmosphere (cont’d)
Called fluorescence
Electrons in N2 gas and O2 gas will be excited
Electrons become promoted to higher sublevel
They have a time-frame for which they can stay excited
Once reached, it may fall down energy sublevels or entire energy levels
Photon is released
F L U O R E S C E N C E
Slide 9
Atmosphere – Fluorescence (cont’d)
Oxygen gas and nitrogen gas bright-line emission spectra
This is [half of the reason] why sky is blue!
Slide 10
Atmosphere (cont’d)
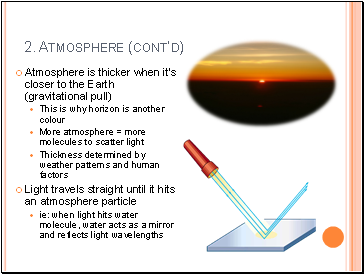
Atmosphere is thicker when it’s closer to the Earth (gravitational pull)
This is why horizon is another colour
More atmosphere = more molecules to scatter light
Thickness determined by weather patterns and human factors
Light travels straight until it hits an atmosphere particle
ie: when light hits water molecule, water acts as a mirror and reflects light wavelengths
Contents
Last added presentations
- Thermal Energy
- Resource Acquisition and Transport in Vascular Plants
- Newton’s laws of motion
- Newton’s Laws of Motion
- Friction
- Radiation Safety and Operations
- Newton's Laws