The Ideal Gas LawPage
1
1
Slide 1
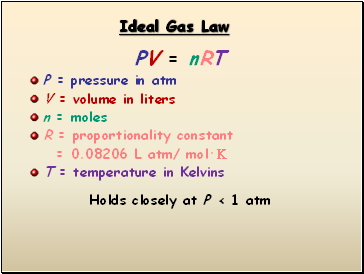
Ideal Gas Law
PV = nRT
P = pressure in atm
V = volume in liters
n = moles
R = proportionality constant
= 0.08206 L atm/ mol·K
T = temperature in Kelvins
Holds closely at P < 1 atm
Slide 2
Ideal Gases
Ideal gases are imaginary gases that perfectly fit all of the assumptions of the kinetic molecular theory.
Gases consist of tiny particles that are far apart
relative to their size.
Collisions between gas particles and between
particles and the walls of the container are
elastic collisions
No kinetic energy is lost in elastic
collisions
Slide 3
Ideal Gases (continued)
Gas particles are in constant, rapid motion. They
therefore possess kinetic energy, the energy of
motion
There are no forces of attraction between gas
particles
The average kinetic energy of gas particles
depends on temperature, not on the identity
of the particle.
Slide 4
Real Gases Do Not Behave Ideally
Real gases DO experience inter-molecular attractions
Real gases DO have volume
Real gases DO NOT have elastic collisions
Slide 5
Deviations from Ideal Behavior
Contents
Last added presentations
- History of Modern Astronomy
- Simulation at NASA for the Space Radiation Effort
- Upcoming Classes
- Soil and Plant Nutrition
- Ch 9 Nuclear Radiation
- Buoyancy
- Friction