Rates of reactionsPage
1
1
Slide 1
Rates of Reactions and Enzymes
Slide 2
Rates of Reaction
Chemical reactions occur when different atoms or molecules collide:
For the reaction to happen the particles must have a certain amount of energy – this is called the ACTIVATION ENERGY.
The rate at which the reaction happens depends on four things:
The temperature of the reactants,
Their concentration
Their surface area
Whether or not a catalyst is used
Slide 3
Measuring rate of reaction
Two common ways:
1) Measure how fast the products are formed
2) Measure how fast the reactants are used up
Slide 4
Rate of reaction graph
Amount of product formed
Time
Slide 5
Enzymes
Enzymes are biological catalysts. They help the reactions that occur in our bodies by controlling the rate of reaction.
Yeast is an example of an enzyme. It is used to help a process called fermentation:
The alcohol from this process is used in making drinks and the carbon dioxide can be used to make bread rise.
Enzymes work best in certain conditions:
Slide 6
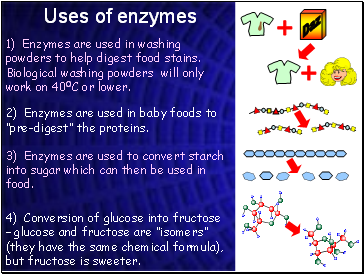
Uses of enzymes
1) Enzymes are used in washing powders to help digest food stains. Biological washing powders will only work on 400C or lower.
2) Enzymes are used in baby foods to “pre-digest” the proteins.
3) Enzymes are used to convert starch into sugar which can then be used in food.
4) Conversion of glucose into fructose – glucose and fructose are “isomers” (they have the same chemical formula), but fructose is sweeter.
Contents
Last added presentations
- Direct heat utilization of geothermal energy
- Madame Marie Curie
- Radiation
- Practical Applications of Solar Energy
- Newton's Laws
- Sound
- Ch 9 Nuclear Radiation