Water and the Fitness of the EnvironmentPage
1
1
Slide 1
The Molecule That Supports All of Life
Water is the biological medium on Earth
All living organisms require water more than any other substance
Most cells are surrounded by water, and cells themselves are about 70–95% water
The abundance of water is the main reason the Earth is habitable
Slide 2
Fig. 3-1
Slide 3
Concept 3.1: The polarity of water molecules results in hydrogen bonding
The water molecule is a polar molecule: The opposite ends have opposite charges
Polarity allows water molecules to form hydrogen bonds with each other
Animation: Water Structure
Slide 4
Fig. 3-2
Hydrogen
bond
–
H
+
H
O
——
——
+
+
+
–
–
–
Slide 5
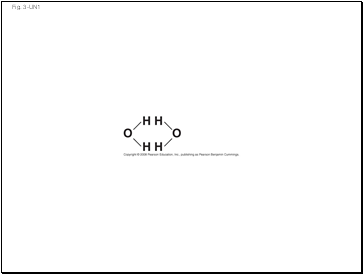
Fig. 3-UN1
Slide 6
Concept 3.2: Four emergent properties of water contribute to Earth’s fitness for life
Four of water’s properties that facilitate an environment for life are:
Cohesive behavior
Ability to moderate temperature
Expansion upon freezing
Versatility as a solvent
Slide 7
Cohesion
Collectively, hydrogen bonds hold water molecules together, a phenomenon called cohesion
Cohesion helps the transport of water against gravity in plants
Adhesion is an attraction between different substances, for example, between water and plant cell walls
Animation: Water Transport
Slide 8
Fig. 3-3
Water-conducting
cells
Adhesion
Cohesion
150 µm
Direction
of water
movement
Slide 9
Surface tension is a measure of how hard it is to break the surface of a liquid
Surface tension is related to cohesion
Slide 10
Fig. 3-4
Slide 11
Moderation of Temperature
Water absorbs heat from warmer air and releases stored heat to cooler air
Water can absorb or release a large amount of heat with only a slight change in its own temperature
Slide 12
Contents
- The Molecule That Supports All of Life
- Cohesion
- Moderation of Temperature
- Insulation of Bodies of Water by Floating Ice
- The Solvent of Life
- Effects of Changes in pH
- Threats to Water Quality on Earth
Last added presentations
- Magnetic field uses sound waves to ignite sun's ring of fire
- Solar Thermal Energy
- Motion
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Ch 9 Nuclear Radiation
- Sound
- The Effects of Radiation on Living Things