Hybridization of OrbitalsPage
3
3
Pi () bonds exist in the region above and below a line drawn between two bonded atoms.
Slide 19
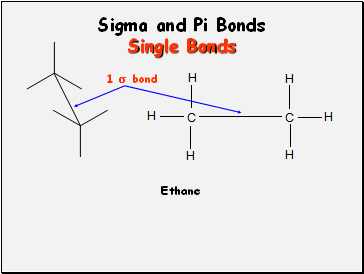
Sigma and Pi Bonds
Single Bonds
Ethane
1 bond
Slide 20
Sigma and Pi Bonds: Double bonds
Ethene
1 bond
1 bond
Slide 21
Sigma and Pi Bonds Triple Bonds
Ethyne
1 bond
1 bond
1 bond
Slide 22
The De-Localized Electron Model
Pi bonds () contribute to the delocalized model of electrons in bonding, and help explain resonance
Electron density from bonds can be distributed symmetrically all around the ring, above and below the plane.
Contents
- Hybridization - The Blending of Orbitals
- What Proof Exists for Hybridization?
- Carbon ground state configuration
- Carbon’s Bonding Problem
- Carbon’s Empty Orbital
- Hybrid Orbitals
- Exclusion Warning
- Hybridization Involving “d” Orbitals
- Hybridization and Molecular Geometry
- Sigma and Pi Bonds
- Sigma and Pi Bonds
- The De-Localized Electron Model
Last added presentations
- Soil and Plant Nutrition
- Mechanics Lecture
- Health Physics
- Radiation
- Newton’s third law of motion
- Heat-Energy on the Move
- Newton’s Laws of Motion
© 2010-2025 powerpoint presentations