Acid-Base. NeutralizPage
1
1
Slide 1
1/26/2017
Chapter 9 Acids and Bases
Acid-Base Neutralization
Buffers
Acid-Base Titration
Slide 2
Neutralization Reactions
1/26/2017
When acid and bases with equal amounts of hydrogen ion H+ and hydroxide ions OH- are mixed, the resulting solution is neutral.
NaOH (aq) + HCl(aq) NaCl + H2O
base acid salt water
Ca(OH)2 + 2 HCl CaCl2 + 2H2O
base acid salt water
Slide 3
Neutralization
1/26/2017
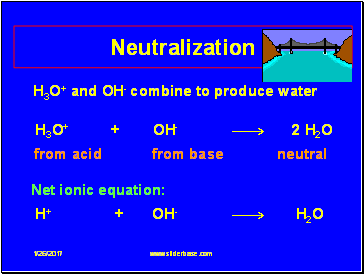
H3O+ and OH- combine to produce water
H3O+ + OH- 2 H2O
from acid from base neutral
Net ionic equation:
H+ + OH- H2O
Slide 4
Ionic Equations for Neutralization
1/26/2017
Write strong acids, bases, and salt as ions
H+ + Cl- + Na+ + OH- Na+ + Cl- + H2O
Cross out matched ions
H+ + Cl- + Na+ + OH- Na+ + Cl- + H2O
Write a net ionic reaction
H+ + OH- H2O
Slide 5
Balancing Neutralization Equations
1/26/2017
Write the equation for the neutralization between magnesium hydroxide and nitric acid. 1. Write the formulas of the acid and base
Mg(OH)2 + HNO3
2. Balance to give equal OH- and H+
Mg(OH)2 + 2 HNO3
Slide 6
1/26/2017
3. Write the products:
Mg(OH)2 + 2HNO3 Mg(NO3)2 + H2O
salt water
(metal and nonmetal)
4. Balance products
Mg(OH)2 + 2 HNO3 Mg(NO3)2 + 2 H2O
Slide 7
Learning Check N2
1/26/2017
Select the correct group of coefficients for the following neutralization equations
A. HCl + Al(OH)3 AlCl3 + H2O
1) 1, 3, 3, 1 2) 3, 1, 1, 1 3) 3, 1, 1 3
B. Ba(OH)2 + H3PO4 Ba3(PO4)2 + H2O
1) 3, 2, 2, 2 2) 3, 3, 1, 6 3) 2, 3, 1, 6
Slide 8
1/26/2017
Solution N2
A. 3HCl + 1Al(OH)3 1AlCl3 + 3H2O
B. 3Ba(OH)2 + 2H3PO4 1Ba3(PO4)2 + 6H2O
Slide 9
1/26/2017
Learning Check N3
Write a balanced equation and calculate the mL of 2.00 M H2SO4 required to neutralize 50.0 mL of 1.00 M KOH?
_H2SO4 + _KOH _K2SO4 + H2O
1) 12.5 mL 2) 50.0 mL 3) 200. mL
Slide 10
1/26/2017
1 2
Contents
- Neutralization Reactions
- Neutralization
- Ionic Equations for Neutralization
- Balancing Neutralization Equations
Last added presentations
- Newton’s third law of motion
- Magnetic field uses sound waves to ignite sun's ring of fire
- Health Physics
- The Effects of Radiation on Living Things
- Geophysical Concepts, Applications and Limitations
- Practical Applications of Solar Energy
- Mechanics Lecture