Acids and BasesPage
4
4
Slide 26

Oxides
Acidic Oxides (Acid Anhydrides): When a covalent oxide dissolves in water an acidic solution forms.
OX bond is strong and covalent.
SO2, NO2, CO2, CrO3
Basic Oxides (Basic Anhydrides): When an ionic oxide dissolves in water a basic solution results.
OX bond is ionic.
K2O, CaO
Slide 27
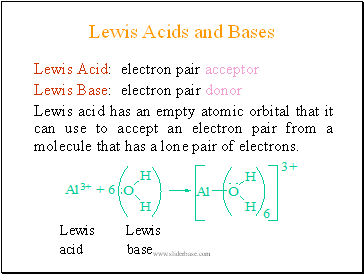
Lewis Acids and Bases
Lewis Acid: electron pair acceptor
Lewis Base: electron pair donor
Lewis acid has an empty atomic orbital that it can use to accept an electron pair from a molecule that has a lone pair of electrons.
Lewis Lewis
acid base
Slide 28

Figure 14.13 The AI(H2O)63+ Ion
Contents
- Acids and Bases
- Conjugate Acid/Base Pairs
- Acid Dissociation Constant (Ka)
- Acid Strength
- Water as an Acid and a Base
- The pH Scale
- Calculating the pH of Strong Acid Solutions
- Solving Weak Acid Equilibrium Problems
- Percent Dissociation (Ionization)
- Bases
- Polyprotic Acids
- Acid-Base Properties of Salts
- Structure and Acid-Base Properties
- Oxides
- Lewis Acids and Bases
Last added presentations
- Newton's Laws
- Practical Applications of Solar Energy
- Gravitation
- History of Modern Astronomy
- Health Physics
- Madame Marie Curie
- Waves & Sound
© 2010-2025 powerpoint presentations
