Acids and BasesPage
1
1
Slide 1
Acids and Bases
Chapter 14
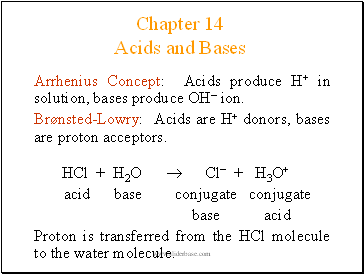
Arrhenius Concept: Acids produce H+ in solution, bases produce OH ion.
Brønsted-Lowry: Acids are H+ donors, bases are proton acceptors.
HCl + H2O Cl + H3O+
acid base conjugate conjugate
base acid
Proton is transferred from the HCl molecule to the water molecule.
Slide 2

Figure 14.1 The Reaction of HCI and H2O
Figure 14.2 The Reaction of an Acid with Water
Figure 14.3 The Reaction of NH3 with HCI to Form NH4+ and CI-
Slide 3
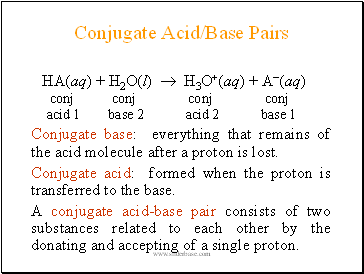
Conjugate Acid/Base Pairs
HA(aq) + H2O(l) H3O+(aq) + A(aq)
conj conj conj conj
acid 1 base 2 acid 2 base 1
Conjugate base: everything that remains of the acid molecule after a proton is lost.
Conjugate acid: formed when the proton is transferred to the base.
A conjugate acid-base pair consists of two substances related to each other by the donating and accepting of a single proton.
Slide 4

Acid Dissociation Constant (Ka)
HA(aq) + H2O(l) H3O+(aq) + A(aq)
Where, Ka is the acid dissociation constant. In dilute solution we can assume that the concentration of liquid water remains essentially constant when an acid is dissolved.
Slide 5
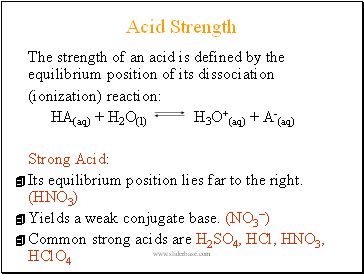
Acid Strength
The strength of an acid is defined by the equilibrium position of its dissociation
(ionization) reaction:
HA(aq) + H2O(l) H3O+(aq) + A-(aq)
Strong Acid:
Its equilibrium position lies far to the right. (HNO3)
Yields a weak conjugate base. (NO3)
Common strong acids are H2SO4, HCl, HNO3, HClO4
Slide 6

Figure 14.4 Graphic Representation of the Behavior of Acids of Different Strengths in Aqueous Solution
Slide 7
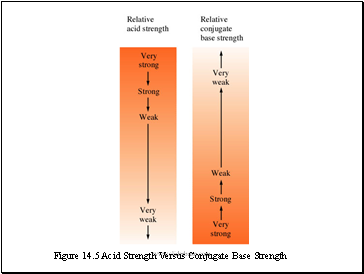
Figure 14.5 Acid Strength Versus Conjugate Base Strength
Slide 8

Acid Strength (continued)
Weak Acid:
Its equilibrium lies far to the left. (CH3COOH)
Yields a much stronger (it is relatively strong) conjugate base than water. (CH3COO)
Common weak acids are H3PO4, HNO2, HOCl, organic acids (-COOH).
Slide 9

Figure 14.6 A Strong Acid (a) and a Weak Acid (b) in Water
Contents
- Acids and Bases
- Conjugate Acid/Base Pairs
- Acid Dissociation Constant (Ka)
- Acid Strength
- Water as an Acid and a Base
- The pH Scale
- Calculating the pH of Strong Acid Solutions
- Solving Weak Acid Equilibrium Problems
- Percent Dissociation (Ionization)
- Bases
- Polyprotic Acids
- Acid-Base Properties of Salts
- Structure and Acid-Base Properties
- Oxides
- Lewis Acids and Bases
Last added presentations
- Solar Thermal Energy
- Newton’s law of universal gravitation
- Motion
- Sound
- Heat-Energy on the Move
- Newton’s third law of motion
- Madame Marie Curie
