Properties of WaterPage
1
1
Slide 1
The Extraordinary Properties of Water
Slide 2
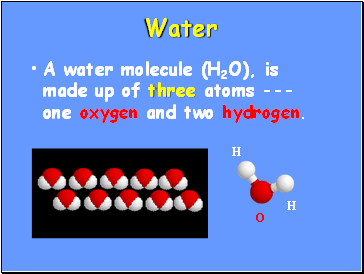
Water
A water molecule (H2O), is made up of three atoms --- one oxygen and two hydrogen.
O
Slide 3
Water is Polar
In each water molecule, the oxygen atom attracts more than its "fair share" of electrons
The oxygen end “acts” negative
The hydrogen end “acts” positive
Causes the water to be POLAR
However, Water is neutral (equal number of e- and p+) --- Zero Net Charge
Slide 4
Hydrogen Bonds Exist Between Water Molecules
Formed between a highly Electronegative atom of a polar molecule and a Hydrogen
One hydrogen bond is weak , but many hydrogen bonds are strong
Slide 5
Interaction Between Water Molecules
Negative Oxygen end of one water molecule is attracted to the Positive Hydrogen end of another water molecule to form a HYDROGEN BOND
Slide 6
What are the Properties of Water?
Slide 7
Properties of Water
At sea level, pure water boils at 100 °C and freezes at 0 °C.
The boiling temperature of water decreases at higher elevations (lower atmospheric pressure).
For this reason, an egg will take longer to boil at higher altitudes
Slide 8
Properties of Water
Cohesion
Slide 9
Properties of Water
Cohesion
Adhesion
Slide 10
Properties of Water
Cohesion
Adhesion
High Specific Heat
Slide 11
Properties of Water
Cohesion
Adhesion
High Specific Heat
High Heat of Vaporization
Slide 12
Properties of Water
Cohesion
Adhesion
High Specific Heat
High Heat of Vaporization
Less Dense as a Solid
Slide 13
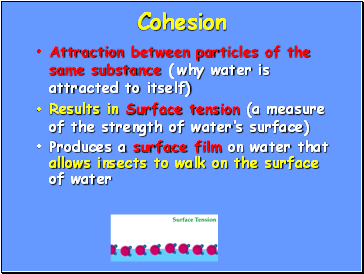
Cohesion
Attraction between particles of the same substance ( why water is attracted to itself)
Results in Surface tension (a measure of the strength of water’s surface)
Contents
- Water
- Water is Polar
- Hydrogen Bonds Exist Between Water Molecules
- Interaction Between Water Molecules
- Properties of Water
- Cohesion
- Adhesion
- Adhesion Causes Capillary Action
- High Specific Heat
- High Heat of Vaporization
- Water is Less Dense as a Solid
- Homeostasis
- Solutions & Suspensions
- Solution
- Suspensions
- Acids
- Bases
- Buffers
Last added presentations
- Heat-Energy on the Move
- Buoyancy
- Newton’s law of universal gravitation
- Solar Thermal Energy
- Newton’s third law of motion
- Resource Acquisition and Transport in Vascular Plants
- Friction