Group 7 HalogensPage
1
1
Slide 1
Group 7 – The Halogens
Slide 2
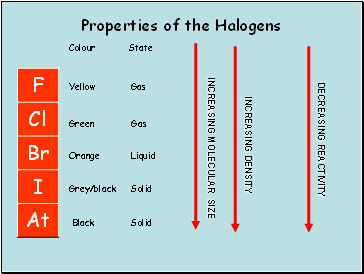
Properties of the Halogens
Colour
Green
Orange
Grey/black
State
Gas
Liquid
Solid
Yellow
Black
Solid
Gas
Slide 3
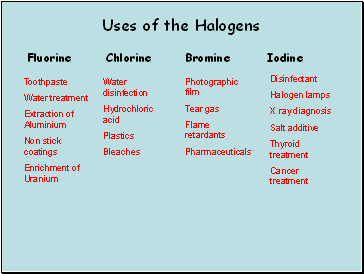
Uses of the Halogens
Fluorine
Iodine
Bromine
Chlorine
Toothpaste
Water treatment
Extraction of Aluminium
Non stick coatings
Enrichment of Uranium
Water disinfection
Hydrochloric acid
Plastics
Bleaches
Photographic film
Tear gas
Flame retardants
Pharmaceuticals
Disinfectant
Halogen lamps
X ray diagnosis
Salt additive
Thyroid treatment
Cancer treatment
Slide 4
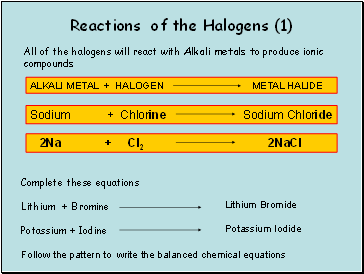
Reactions of the Halogens
All of the halogens will react with Alkali metals to produce ionic compounds
Lithium Bromide
Potassium Iodide
Follow the pattern to write the balanced chemical equations
Slide 5
Reactions of the Halogens (2)
All of the halogens will react with Hydrogen to produce gasas
Hydrogen Bromide
Hydrogen Iodide
Follow the pattern to write the balanced chemical equations
Slide 6
Reactions of the Halogens (3)
All of the halogens will react with water to produce 2 acids
This also happens with Bromine and Iodine and the acids formed are much stronger!
Contents
Last added presentations
- Direct heat utilization of geothermal energy
- Radiation
- Sound
- Sound
- Heat-Energy on the Move
- Newton's Laws
- Newton's laws of motion