Chemical EquilibriumPage
1
1
Slide 1
Chemical equilibrium
Cato Maximilian Guldberg and his brother-in-law Peter Waage developed the Law of Mass Action
Slide 2
Chemical Equilibrium
Reversible Reactions:
A chemical reaction in which the products
can react to re-form the reactants
Chemical Equilibrium:
When the rate of the forward reaction
equals the rate of the reverse reaction
and the concentration of products and
reactants remains unchanged
2HgO(s) 2Hg(l) + O2(g)
Arrows going both directions ( ) indicates equilibrium in a chemical equation
Slide 3
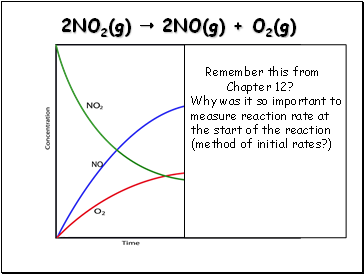
2NO2(g) 2NO(g) + O2(g)
Remember this from
Chapter 12?
Why was it so important to measure reaction rate at the start of the reaction
(method of initial rates?)
Slide 4
2NO2(g) 2NO(g) + O2(g)
Slide 5
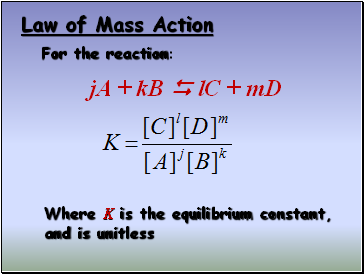
Law of Mass Action
For the reaction:
Where K is the equilibrium constant, and is unitless
jA + kB lC + mD
Slide 6
Product Favored Equilibrium
Large values for K signify the reaction is “product favored”
When equilibrium is achieved, most reactant has been converted to product
Slide 7
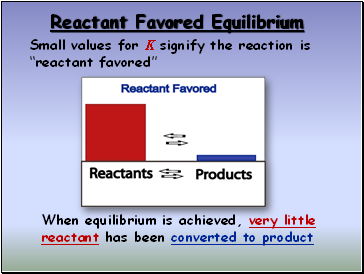
Reactant Favored Equilibrium
Small values for K signify the reaction is “reactant favored”
When equilibrium is achieved, very little reactant has been converted to product
Slide 8
Writing an Equilibrium Expression
2NO2(g) 2NO(g) + O2(g)
K = ???
Write the equilibrium expression for the reaction:
Slide 9
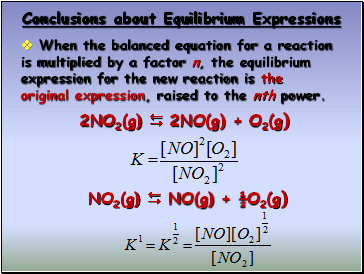
Conclusions about Equilibrium Expressions
The equilibrium expression for a reaction is the reciprocal for a reaction written in reverse
2NO2(g) 2NO(g) + O2(g)
2NO(g) + O2(g) 2NO2(g)
Slide 10
Conclusions about Equilibrium Expressions
When the balanced equation for a reaction is multiplied by a factor n, the equilibrium expression for the new reaction is the original expression, raised to the nth power.
2NO2(g) 2NO(g) + O2(g)
NO2(g) NO(g) + ½O2(g)
Slide 11
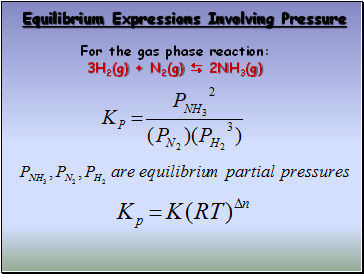
Equilibrium Expressions Involving Pressure
1 2
Contents
- Chemical equilibrium
- Law of Mass Action
- Product Favored Equilibrium
- Reactant Favored Equilibrium
- Writing an Equilibrium Expression
- Conclusions about Equilibrium Expressions
- Equilibrium Expressions Involving Pressure
- Heterogeneous Equilibria
- The Reaction Quotient
- Significance of the Reaction Quotient
- Solving for Equilibrium Concentration
Last added presentations
- Buoyancy
- Thermal Energy
- Practical Applications of Solar Energy
- Geophysical Concepts, Applications and Limitations
- Newton’s Law of Gravity
- Newton’s laws of motion
- Newton's laws of motion