Carboxylic acidsPage
1
1
Slide 1
Alcohols revisited (and ethers)
and going further .
13.3 Carboxylic acids and their derivatives
Slide 2
Alcohols
homologous series containing the OH hydroxyl group.
all names end in ol eg methanol, ethanol etc.
isomers are possible for alcohols containing 3 or more carbons.
label position of OH group so that it has the lowest number possible.
polyhydric alcohols contain more than one OH group eg propane- 1,2,3,triol
OH groups attached to benzene rings are called phenols.
Slide 3
Physical properties of alcohols
Molecules are polar, in the O-H bond, O is - and H is +
Molecules have attractive forces between the molecules called hydrogen bonds, not as strong as covalent bonds.
Higher boiling point than corresponding alkanes.
Hydrogen bonds form between alcohol and water molecules therefore they are miscible / soluble.
Long chain alcohols are less soluble.
Slide 4
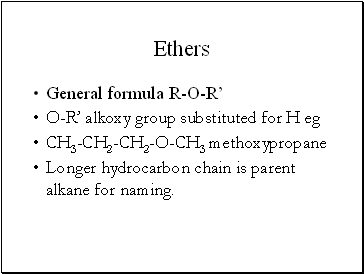
Ethers
General formula R-O-R’
O-R’ alkoxy group substituted for H eg
CH3-CH2-CH2-O-CH3 methoxypropane
Longer hydrocarbon chain is parent alkane for naming.
Slide 5
Physical properties of ethers
Molecules only slightly polar.
No hydrogen on the oxygen atoms to form hydrogen bonds – only weak forces of attraction between molecules.
Boiling points similar to corresponding alkane.
Lower ethers, very volatile, highly flammable.
Only slightly soluble in water, mix well with other non-polar solvents eg alkanes. (Like dissolves like).
Slide 6
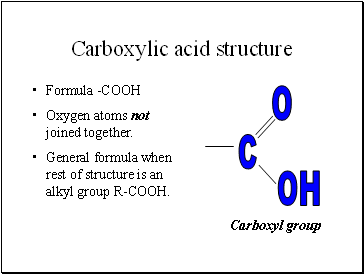
Carboxylic acid structure
Formula -COOH
Oxygen atoms not joined together.
General formula when rest of structure is an alkyl group R-COOH.
Carboxyl group
Slide 7
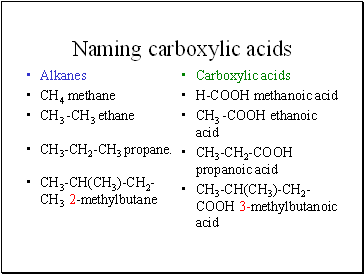
Naming carboxylic acids
Alkanes
CH4 methane
CH3 -CH3 ethane
CH3-CH2-CH3 propane.
CH3-CH(CH3)-CH2-CH3 2-methylbutane
Carboxylic acids
H-COOH methanoic acid
CH3 -COOH ethanoic acid
CH3-CH2-COOH propanoic acid
CH3-CH(CH3)-CH2-COOH 3-methylbutanoic acid
Slide 8
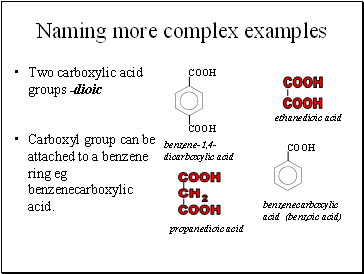
Naming more complex examples
Two carboxylic acid groups -dioic
Carboxyl group can be attached to a benzene ring eg benzenecarboxylic acid.
ethanedioic acid
propanedioic acid
benzenecarboxylic acid (benzoic acid)
1 2
Contents
- Alcohols
- Physical properties of alcohols
- Ethers
- Physical properties of ethers
- Carboxylic acid structure
- Naming carboxylic acids
- Naming more complex examples
- Carboxylic acid derivatives
- Naming practise.
Last added presentations
- Space Radiation
- Newton’s third law of motion
- Geophysical Concepts, Applications and Limitations
- Sound
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- The Effects of Radiation on Living Things
- Newton's Laws