Calculations of Solution ConcentrationPage
1
1
Slide 1
Calculations of Solution Concentration
Slide 2
CA Standards
Slide 3
Calculations of Solution Concentration
Grams per Liter
Grams per liter is the ratio of mass units of solute to volume (liters) of solution
Slide 4
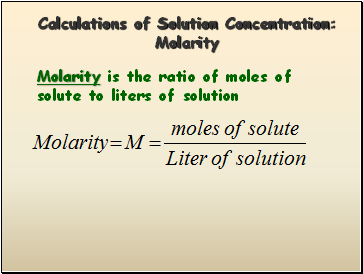
Calculations of Solution Concentration: Molarity
Molarity is the ratio of moles of solute to liters of solution
Slide 5
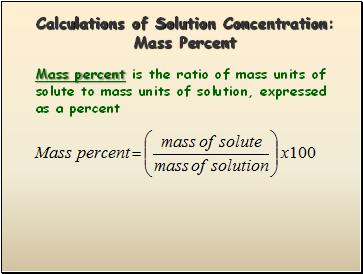
Calculations of Solution Concentration: Mass Percent
Mass percent is the ratio of mass units of solute to mass units of solution, expressed as a percent
Slide 6
Calculations of Solution Concentration: Parts per Million
Parts per million is the ratio of mass units of solute to mass units of solution, multiplied by one million (106)
Slide 7
A Simplifying Assumption
1 ml of water = 1 gram of water
1000 ml of water = 1 liter = 1000 grams
Assume that solutions with water as the solvent have the density of pure water (1 mL = 1 gram)
Itís not true, but itís close enough
Contents
Last added presentations
- Radiation Safety and Operations
- Newton's laws of motion
- Direct heat utilization of geothermal energy
- Mechanics Lecture
- Motion
- Practical Applications of Solar Energy
- Simulation at NASA for the Space Radiation Effort