Atoms and moleculesPage
1
1
Slide 1
More about atoms and molecules
Slide 2
What is an atom mostly made of?
A) The nucleus
B) Electrons
C) Empty space (nothing)
Slide 3
What is an atom mostly made of?
A) The nucleus
B) Electrons
C) Empty space (nothing)
Slide 4
The sizes of things in an atom
The nucleus of an atom is very small compared to the size of the atom
Electrons are also very small compared to protons and neutrons
If a proton were the size of a marble, an electron would be about the width of a human hair, and the electron would be about 4 kilometers away from the proton.
Slide 5
The Rutherford Model of the Atom
The “Rutherford Model” of the atom is a simple way of thinking of an atom
It is not correct in some ways
In the Rutherford Model, electrons move around (orbit) the nucleus like planets moving around the sun
Slide 6
Probability
Probability is the chance that something will happen
It is a number between 0 (definitely won’t happen) to 1 (definitely will happen)
If I roll a 6-sided die, the probability of rolling a 6 is 1/6 (0.167). It is the same probability for rolling any of the numbers on the die.
Slide 7
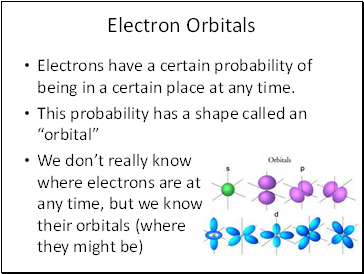
Electron Orbitals
Electrons have a certain probability of being in a certain place at any time.
This probability has a shape called an “orbital”
We don’t really know where electrons are at any time, but we know their orbitals (where they might be)
Slide 8
Ions
An ion is an atom or molecule in which the # of electrons is not equal to the # of protons
This gives the atom an overall positive or negative electrical charge
If it has a positive charge its called a cation.
If it has a negative charge its called an anion.
Slide 9
Avogadro Constant and Mole
“Avogadro constant” is a number, and is about 6.022×1023 or 602,200,000,000,000,000,000,000
A mole is the amount of any substance that has an Avogadro’s constant (6.022×1023) atoms or molecules
“Mole” is also the name of an animal that lives underground
Slide 10
How to use a mole
1 2
Contents
- More about atoms and molecules
- What is an atom mostly made of?
- The sizes of things in an atom
- The Rutherford Model of the Atom
- Probability
- Electron Orbitals
- Ions
- Avogadro Constant and Mole
- How to use a mole
- Problems with moles
Last added presentations
- Friction
- Space Radiation
- Solar Thermal Energy
- Newton’s Law of Gravity
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Radioactivity and Nuclear Reactions
- Solar Energy