Acids, pH and EquilibriumPage
1
1
Slide 1
Acid Equilibrium and pH
Søren Sørensen
Slide 2
Acid/Base Definitions
Arrhenius Model
Acids produce hydrogen ions in aqueous solutions
Bases produce hydroxide ions in aqueous solutions
Bronsted-Lowry Model
Acids are proton donors
Bases are proton acceptors
Lewis Acid Model
Acids are electron pair acceptors
Bases are electron pair donors
Slide 3
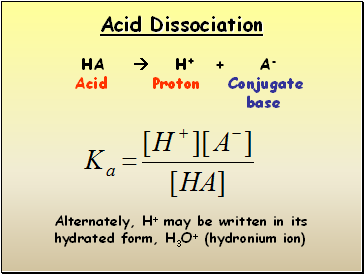
Acid Dissociation
HA H+ + A-
Acid Proton Conjugate
base
Alternately, H+ may be written in its hydrated form, H3O+ (hydronium ion)
Slide 4
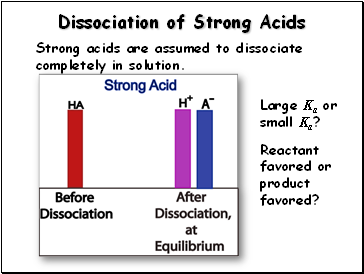
Dissociation of Strong Acids
Strong acids are assumed to dissociate completely in solution.
Large Ka or small Ka?
Reactant favored or product favored?
Slide 5
Dissociation Constants: Strong Acids
Slide 6
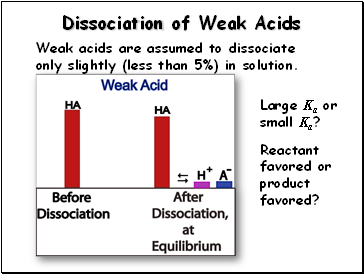
Dissociation of Weak Acids
Weak acids are assumed to dissociate only slightly (less than 5%) in solution.
Large Ka or small Ka?
Reactant favored or product favored?
Slide 7
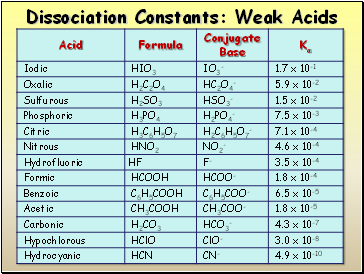
Dissociation Constants: Weak Acids
Slide 8
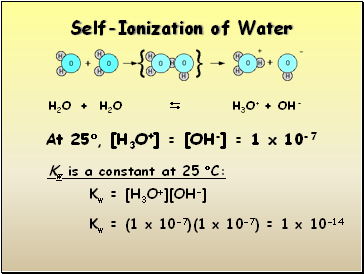
Self-Ionization of Water
H2O + H2O H3O+ + OH-
At 25, [H3O+] = [OH-] = 1 x 10-7
Kw is a constant at 25 C:
Kw = [H3O+][OH-]
Kw = (1 x 10-7)(1 x 10-7) = 1 x 10-14
Slide 9
Calculating pH, pOH
pH = -log10(H3O+)
pOH = -log10(OH-)
Relationship between pH and pOH
pH + pOH = 14
Finding [H3O+], [OH-] from pH, pOH
[H3O+] = 10-pH
[OH-] = 10-pOH
Slide 10
pH and pOH Calculations
Slide 11
The pH Scale
Graphic: Wikimedia Commons user Slower
Slide 12
A Weak Acid Equilibrium Problem
What is the pH of a 0.50 M solution of acetic acid, HC2H3O2, Ka = 1.8 x 10-5 ?
Step #1: Write the dissociation equation
HC2H3O2 C2H3O2- + H+
Slide 13
1 2
Contents
- Acid Equilibrium and pH
- Acid/Base Definitions
- Acid Dissociation
- Dissociation of Strong Acids
- Dissociation Constants: Strong Acids
- Dissociation of Weak Acids
- Dissociation Constants: Weak Acids
- Self-Ionization of Water
- A Weak Acid Equilibrium Problem
Last added presentations
- Soil and Plant Nutrition
- Friction
- Solar Thermal Energy
- Radiation Safety and Operations
- Heat-Energy on the Move
- Radioactivity and Nuclear Reactions
- History of Modern Astronomy