Acid, bases and saltsPage
1
1
Slide 1
Acids, Bases & Salts
Slide 2
Terms
ACIDS are substances that form hydrogen ions (H+(aq)) when dissolved in water eg
Hydrochloric acid HCl gives H+(aq) and Cl-(aq) ions,
Sulphuric acid H2SO4 gives 2H+(aq) and SO42- ions
Nitric acid HNO3 gives H+(aq) and NO3-(aq) ions.
BASES are oxides and hydroxides of metals that react and neutralise acids to form salts and water only. Bases which are soluble in water are called alkalis. Not all bases fit into these categories e.g. ammonia.
Alkalis are substances that form hydroxide ions OH-(aq) in water eg
Sodium Hydroxide NaOH gives Na+(aq) and OH-(aq) ions,
Calcium Hydroxide Ca(OH)2 gives Ca2+(aq) and 2OH-(aq) ions.
Slide 3
In acid solutions there are more H+ ions than OH- ions.
In alkaline solution there are more OH- ions than H+ ions.
Acids that dissociate (ionize) to a large extent are strong electrolytes and Strong Acids.
Acids that dissociate only to a small extent are Weak Acids and weak electrolytes
Bases can be strong or weak depending on the extent to which they dissociate and produce OH– ions in solution. Most metal hydroxides are strong electrolytes and Strong Bases. Ammonia, NH3, is a weak electrolyte and Weak Base.
Slide 4
Basicity of Acid
It is the number of ionizable H+ ions present in an acid e.g.
HCl is mono basic, it ionizes to produce one H+ ion
HCl ============== H+ + Cl-
H2SO4 is Dibasic, It ionizes to produce two H+ ions.
H2SO4 =============== 2H+ + SO42-
H3PO4 is Tribasic, it ionizes to produce three H+ ions.
H3PO4 ============== 3H+ + PO43-
Slide 5
Acidity of a Base
It is the ionizable OH- ions present in an alkali. e.g.
NaOH is monoacidic
NaOH ========== Na+ + OH-
Ca(OH)2 is diacidic
Ca(OH)2 ============== Ca2+ + 2OH-
Slide 6
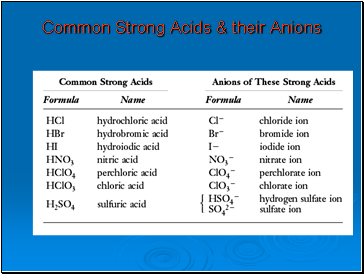
Common Strong Acids & their Anions
Slide 7
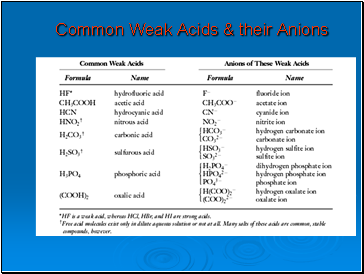
Common Weak Acids & their Anions
Slide 8
Naming of Acids
Binary Acids (H+ and a nonmetal)
hydro (nonmetal) -ide + ic acid
HCl (aq) = hydrochloric acid
Ternary Acids (H+ and a polyatomic ion)
(polyatomic ion) -ate +ic acid
Contents
- Acids, Bases & Salts
- Terms
- Basicity of Acid
- Acidity of a Base
- Common Strong Acids & their Anions
- Common Weak Acids & their Anions
- Naming of Acids
- Formula Writing of Acids
- Properties of Bases
- Naming of Bases
- Formula Writing of Bases
- Physical Properties of Acids & Bases
- Chemical Properties of Acids
- Neutralization
- Formation of Hydronium ion( H30+).
- Uses of Acids
- Chemical Properties of Bases
- Chemical Properties of Bases
- Types of Oxides
- Periodic trends in oxides
- Salts
- Methods of making Soluble Salts
- Making Insoluble Salts
- Precipitation reaction
- Types of Salts
- Hydrated & anhydrous salts
- Uses of salts
- Self Ionization of Water
- The pH Scale
- Indicators.
- pH Graph
- Ionic equations
- Scheme for ionic equation
Last added presentations
- Mechanics Lecture
- Newton’s laws of motion
- Simulation at NASA for the Space Radiation Effort
- Newton's Laws
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Magnetic field uses sound waves to ignite sun's ring of fire
- Newton’s law of universal gravitation