VSEPR and Molecular GeometryPage
1
1
Slide 1
VSEPR and Molecular Geometry
Hemoglobin
Slide 2
Models
Models are attempts to explain how nature operates on the microscopic level based on experiences in the macroscopic world.
Models can be physical as with this DNA model
Models can be mathematical
Models can be theoretical or philosophical
Slide 3
Fundamental Properties of Models
A model does not equal reality.
Models are oversimplifications, and are therefore often wrong.
Models become more complicated as they age.
We must understand the underlying assumptions in a model so that we don’t misuse it.
Slide 4
VSEPR Model
The structure around a given atom is determined principally by minimizing electron pair repulsions.
(Valence Shell Electron Pair Repulsion)
Slide 5
Predicting a VSEPR Structure
Draw Lewis structure.
Put pairs as far apart as possible.
Determine positions of atoms from the way electron pairs are shared
Determine the name of molecular structure from positions of the atoms.
Slide 6
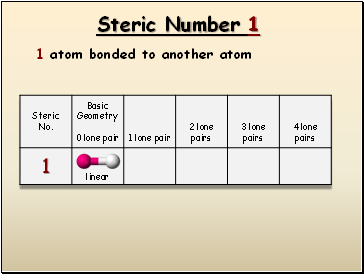
Steric Number
1
1 atom bonded to another atom
Slide 7
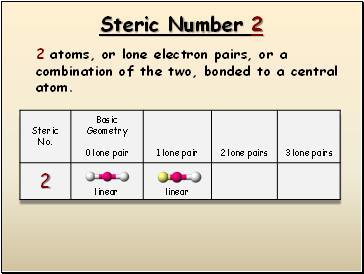
Steric Number 2
2 atoms, or lone electron pairs, or a combination of the two, bonded to a central atom.
Slide 8
Steric Number 3
3 atoms, or lone electron pairs, or a combination of the two, bonded to a central atom.
Slide 9
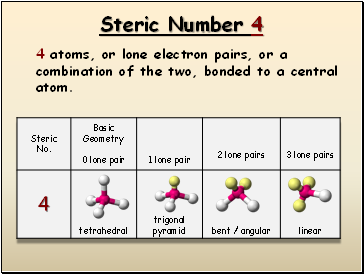
Steric Number 4
4 atoms, or lone electron pairs, or a combination of the two, bonded to a central atom.
Slide 10
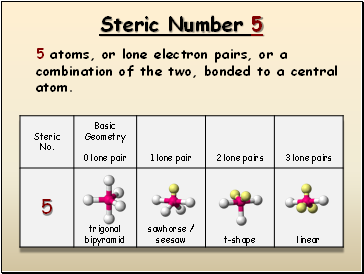
Steric Number 5
5 atoms, or lone electron pairs, or a combination of the two, bonded to a central atom.
Slide 11
Steric Number 6
6 atoms, or lone electron pairs, or a combination of the two, bonded to a central atom.
Slide 12
Steric Number 7
7 atoms, or lone electron pairs, or a combination of the two, bonded to a central atom.
Contents
Last added presentations
- Newton’s law of universal gravitation
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Newton’s third law of motion
- Friction
- Practical Applications of Solar Energy
- Radiation
- Simulation at NASA for the Space Radiation Effort