The MolePage
1
1
Slide 1
The Mole
Slide 2
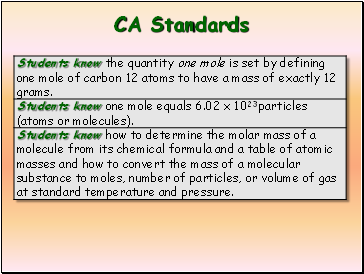
CA Standards
Slide 3
The Mole
1 dozen =
1 gross =
1 ream =
1 mole =
12
144
500
6.02 x 1023
There are exactly 12 grams of carbon-12 in one mole of carbon-12.
Slide 4
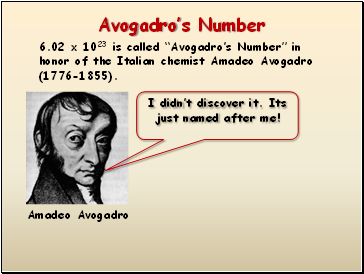
Avogadro’s Number
6.02 x 1023 is called “Avogadro’s Number” in honor of the Italian chemist Amadeo Avogadro (1776-1855).
Amadeo Avogadro
I didn’t discover it. Its just named after me!
Slide 5
Calculating Formula Mass
Calculate the formula mass of carbon dioxide, CO2.
12.01 g + 2(16.00 g) =
44.01 g
One mole of CO2 (6.02 x 1023 molecules) has a mass of 44.01 grams
Slide 6
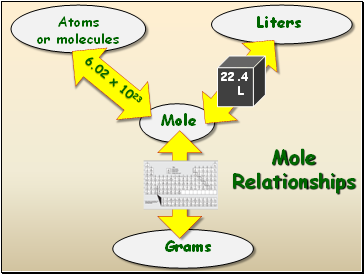
Mole Relationships
Mole
Atoms
or molecules
Liters
Grams
6.02 x 1023
Atomic
Mass
22. 4 L
22.4
L
Slide 7
Calculations with Moles: Converting moles to grams
How many grams of lithium are in 3.50 moles of lithium?
3.50 mol Li
= g Li
1 mol Li
6.94 g Li
24.3
Slide 8
Calculations with Moles: Converting grams to moles
How many moles of lithium are in 18.2 grams of lithium?
18.2 g Li
= mol Li
6.94 g Li
1 mol Li
2.62
Slide 9
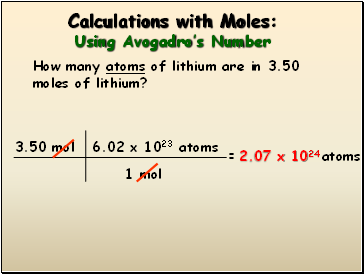
Calculations with Moles: Using Avogadro’s Number
How many atoms of lithium are in 3.50 moles of lithium?
3.50 mol
= atoms
1 mol
6.02 x 1023 atoms
2.07 x 1024
Slide 10
Calculations with Moles: Using Avogadro’s Number
How many atoms of lithium are in 18.2 g of
lithium?
18.2 g Li
= atoms Li
1 mol Li
6.022 x 1023 atoms Li
1.58 x 1024
6.94 g Li
1 mol Li
(18.2)(6.022 x 1023)/6.94
Slide 11
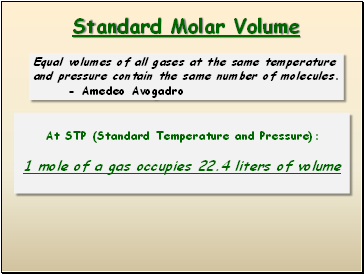
Standard Molar Volume
Equal volumes of all gases at the same temperature and pressure contain the same number of molecules.
- Amedeo Avogadro
At STP (Standard Temperature and Pressure):
1 mole of a gas occupies 22.4 liters of volume
Slide 12
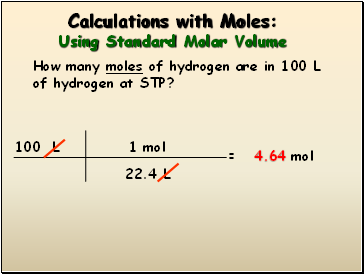
Calculations with Moles: Using Standard Molar Volume
1 2
Contents
- The Mole
- Avogadro’s Number
- Calculating Formula Mass
- Calculations with Moles: Converting moles to grams
- Calculations with Moles: Converting grams to moles
- Calculations with Moles: Using Avogadro’s Number
- Standard Molar Volume
- Calculations with Moles: Using Standard Molar Volume
Last added presentations
- Geophysical Concepts, Applications and Limitations
- Heat-Energy on the Move
- Space Radiation
- Newton’s laws of motion
- Practical Applications of Solar Energy
- Resource Acquisition and Transport in Vascular Plants
- Solar Energy