Light and electromagnetic spectrumPage
1
1
Slide 1
Light and the Electromagnetic Spectrum
Slide 2
Light Phenomenon
Isaac Newton (1642-1727) believed light consisted of particles
By 1900 most scientists believed that light behaved as a wave.
Slide 3
The Electromagnetic Spectrum
The electromagnetic spectrum represents the range of energy from low energy, low frequency radio waves with long wavelengths up to high energy, high frequency gamma waves with small wavelengths.
Slide 4
Visible light is a small portion of this spectrum. This is the only part of this energy range that our eyes can detect. What we see is a rainbow of colors.
RedOrangeYellowGreenBlueIndigoViolet
ROY G BIV
Slide 5
Frequency Ranges
Wavelengths
104 101 1 10-2 10-5 10-6 10-8 10-10 10-12
Frequencies (cycles per sec)
3 x 106 3 x 1010 3 x 1014 3 x 1016 3 x1018 3 x10 22
Slide 6
Frequency Ranges of Visible Light
Red light has a frequency of roughly
4.3 × 1014 Hz, and a wavelength of about 7.0 × 107 m (700nm).
Violet light, at the other end of the visible range, has nearly double the frequency—7.5 × 1014 Hz—and (since the speed of light is the same in either case) just over half the wavelength—
4.0 × 107 m (400nm).
Slide 7
The radiation to which our eyes are most sensitive has a wavelength near the middle of this range, at about
5.5 x 10-7m (550 nm), in the yellow-green region of the spectrum.
Slide 8
It is no coincidence that this wavelength falls within the range of wavelengths at which the Sun emits most of its electromagnetic energy—our eyes have evolved to take greatest advantage of the available light.
Slide 9
C = λν
The frequency (v) of a wave is the number of waves to cross a point in 1 second (units are Hertz – cycles/sec or sec-1)
λ is the wavelength- the distance from crest to crest on a wave
Slide 10
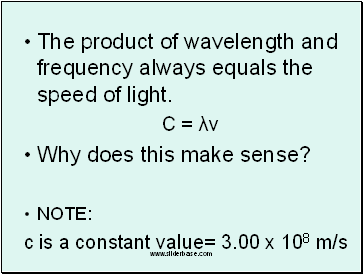
The product of wavelength and frequency always equals the speed of light.
C = λν
Why does this make sense?
Contents
Last added presentations
- Resource Acquisition and Transport in Vascular Plants
- Radiation Safety and Operations
- Space Radiation
- Health Physics
- Newton's laws of motion
- Magnetic field uses sound waves to ignite sun's ring of fire
- History of Modern Astronomy