Nuclear Fission and FusionPage
1
1
Slide 1
Fission and Fusion
Slide 2
Energy and Mass
Nuclear changes occur with small but measurable losses of mass. The lost mass is called the mass defect, and is converted to energy according to Einsteinís equation:
DE = Dmc2
Dm = mass defect
DE = change in energy
c = speed of light
Because c2 is so large, even small amounts of mass are converted to enormous amount of energy.
Slide 3
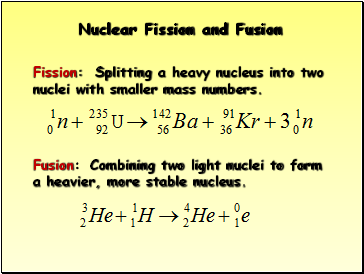
Nuclear Fission and Fusion
Fusion: Combining two light nuclei to form a heavier, more stable nucleus.
Fission: Splitting a heavy nucleus into two nuclei with smaller mass numbers.
Slide 4
Fission
A uranium-235 atom absorbs a neutron, and fissions into two new atoms (fission fragments), releasing three new neutrons and some binding energy.
One of those neutrons is absorbed by an atom of uranium-238, and does not continue the reaction. Another neutron is simply lost and does not collide with anything, also not continuing the reaction. However one neutron does collide with an atom of uranium-235, which then fissions and releases two neutrons and some binding energy.
Both of those neutrons collide with uranium-235 atoms, each of which fission and release between one and three neutrons, and so on.
Source: Wikimedia Commons
Slide 5
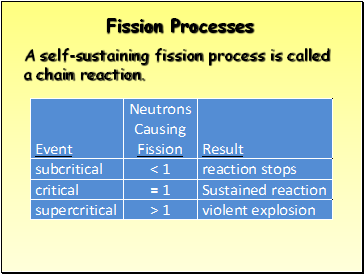
Fission Processes
A self-sustaining fission process is called a chain reaction.
Slide 6
Critical Mass
A sphere of fissile material is too small to allow the chain reaction to become self-sustaining as neutrons generated by fissions can too easily escape.
By increasing the mass of the sphere to a critical mass, the reaction can become self-sustaining
Surrounding the original sphere with a neutron reflector (such as tungsten carbide) increases the efficiency of the reactions and also allows the reaction to become self-sustaining.
Source: Wikipedia
Graphic: Wikimedia Commons
Slide 7
Fission Bomb Design
Fat Man
Little Boy
Slide 8
A Fission Reactor
Slide 9
Fusion
The deuterium-tritium fusion reaction
Slide 10
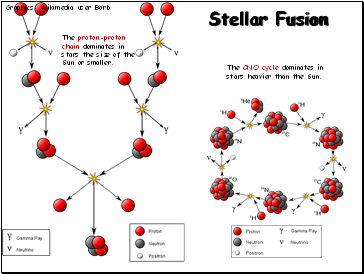
Stellar Fusion
1 2
Contents
- Energy and Mass
- Nuclear Fission and Fusion
- Fission
- Fission Processes
- Fission Bomb Design
- A Fission Reactor
- Fusion
- Stellar Fusion
Last added presentations
- Soil and Plant Nutrition
- Resource Acquisition and Transport in Vascular Plants
- Solar Energy
- Upcoming Classes
- Sensory and Motor Mechanisms
- The Effects of Radiation on Living Things
- Thermal Energy