Mass SpectrometryPage
1
1
Slide 1
Mass Spectrometry
Slide 2
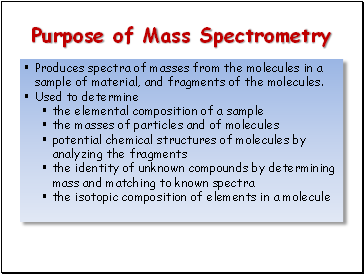
Purpose of Mass Spectrometry
Produces spectra of masses from the molecules in a sample of material, and fragments of the molecules.
Used to determine
the elemental composition of a sample
the masses of particles and of molecules
potential chemical structures of molecules by analyzing the fragments
the identity of unknown compounds by determining mass and matching to known spectra
the isotopic composition of elements in a molecule
Slide 3
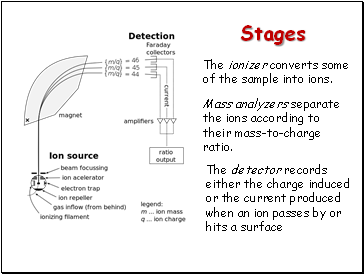
Stages
The ionizer converts some of the sample into ions.
Mass analyzers separate the ions according to their mass-to-charge ratio.
The detector records either the charge induced or the current produced when an ion passes by or hits a surface
Slide 4
Mass Spectrometry Methods
http://www.chem.arizona.edu/massspec/intro_html/intro.html
Slide 5
Mass Spectrum of CO2
Molecular ion peak
[CO2]+ = 44
[CO]+ = 28
[O]+ = 16
[C]+ = 12
Fragment Peaks
Slide 6
Mass Spectrum of Bromine, Br2
Bromine has two isotopes: 50.69% 79Br and 49.31% 81Br
79Br+
81Br+
[79Br81Br]+
[79Br79Br]+
[81Br81Br]+
Molecular Ion Peaks
Fragments
Slide 7
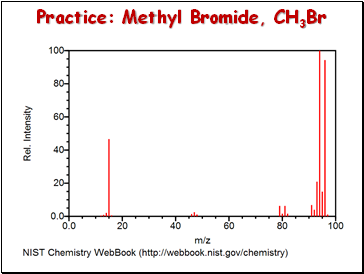
Practice: Methyl Bromide, CH3Br
Slide 8
Answers: Methyl Bromide, CH3Br
[CH381Br]+
[CH281Br]+
[C81Br]+ and [CH279Br]+
[CH81Br]+ and [CH379Br]+
[CH79Br]+
[CH3]+
[C79Br]+
[81Br]+
[79Br]+
Slide 9
Practice: Methylene Chloride (CH2Cl2)
Chlorine is 75.77% 35Cl and 24.23% 37Cl
Slide 10
Practice: Vinyl Chloride (CH2CHCl)
Chlorine is 75.77% 35Cl and 24.23% 37Cl
Slide 11
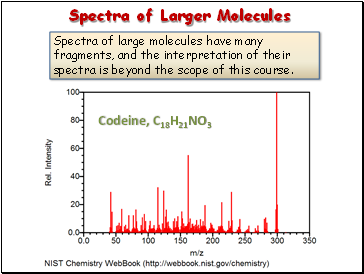
Spectra of Larger Molecules
Spectra of large molecules have many fragments, and the interpretation of their spectra is beyond the scope of this course.
Codeine, C18H21NO3
Slide 12
Toluene ( C7H8) Fragmentation
1 2
Contents
- Purpose of Mass Spectrometry
- Stages
- Mass Spectrometry Methods
- Mass Spectrum of CO2
- Mass Spectrum of Bromine, Br2
- Practice: Methyl Bromide, CH3Br
- Answers: Methyl Bromide, CH3Br
- Spectra of Larger Molecules
- Toluene ( C7H8) Fragmentation
- Mass Spectrometry in Forensics
Last added presentations
- Sound
- Sensory and Motor Mechanisms
- Newton’s Laws of Motion
- Sound
- Static and Kinetic Friction
- Upcoming Classes
- Simulation at NASA for the Space Radiation Effort