Making ElectricityPage
1
1
Slide 1
Making Electricity
electrons.
Electricity passing along metal wires is a flow of
In a cell/battery, electricity comes from a chemical reaction
chemical energy
electrical energy.
Cells/batteries need replaced as the chemicals
are being used up in the reaction to supply electricity.
Some cells/batteries are rechargeable, e.g.
nicad cells (nickel-cadmium cells) and
the lead-acid battery used in cars/vans/buses.
Slide 2
Dry Cells
metal cap
zinc case
carbon rod
(graphite)
ammonium chloride
The ammonium chloride in the cell is an example of an
The purpose of the electrolyte is to
electrolyte.
complete the circuit.
Slide 3
Electricity can be produced by connecting different metals together (with an electrolyte) to form a
cell.
Different pairs of metals connected in a cell give different voltages. This enables us to construct an
electrochemical series (see data booklet - page 7)
Slide 4
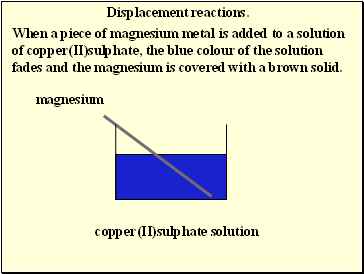
Displacement reactions.
When a piece of magnesium metal is added to a solution of copper(II)sulphate, the blue colour of the solution fades and the magnesium is covered with a brown solid.
magnesium
copper(II)sulphate solution
Slide 5
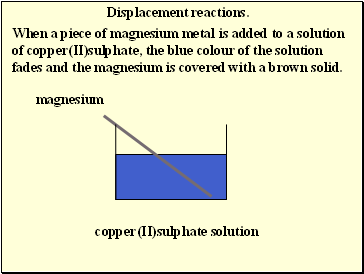
Displacement reactions.
When a piece of magnesium metal is added to a solution of copper(II)sulphate, the blue colour of the solution fades and the magnesium is covered with a brown solid.
magnesium
copper(II)sulphate solution
Slide 6
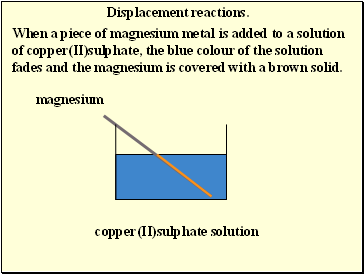
Displacement reactions.
When a piece of magnesium metal is added to a solution of copper(II)sulphate, the blue colour of the solution fades and the magnesium is covered with a brown solid.
magnesium
copper(II)sulphate solution
Slide 7
Displacement reactions.
When a piece of magnesium metal is added to a solution of copper(II)sulphate, the blue colour of the solution fades and the magnesium is covered with a brown solid.
magnesium
copper(II)sulphate solution
Slide 8
Displacement reactions.
When a piece of magnesium metal is added to a solution of copper(II)sulphate, the blue colour of the solution fades and the magnesium is covered with a brown solid.
magnesium
copper(II)sulphate solution
Contents
- Making Electricity
- Dry Cells
- Displacement reactions.
- Cells/batteries compared to mains electricity.
- Oxidation and Reduction
Last added presentations
- Heat-Energy on the Move
- History of Modern Astronomy
- Madame Marie Curie
- Newton’s Law of Gravity
- Newton’s third law of motion
- Mechanics Lecture
- Thermal Energy