The Sun and Its Ability to Create PowerPage
1
1
Slide 1
Chapter 15: The Sun: A Nuclear Powerhouse
Slide 2
Happy Sun
Slide 3
Why Does the Sun Shine?
The Sun gives off energy (duh)!
The energy must come from somewhere - thereís no free lunch.
Conservation of energy is a fundamental tenet of physics.
Where does the energy come from?
Until the 20th century only 2 possibilities were known:
Chemical reactions
Gravity
Slide 4
The Sunís Energy Output
How bright is the Sun?
The Sun produces 4x1026 watts
Watt is the unit for the rate of energy use, commonly seen on light bulbs and appliances.
Our largest power plants produce around 5 x 109 watts of power (5,000 megawatts or 5 gigawatts)
Sun = 8 x 1016 of these power plants (80,000 trillion)
Anyway you look at it, the Sun gives off a lot of energy.
Slide 5
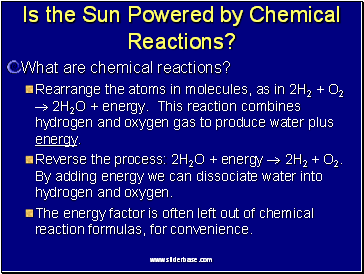
Is the Sun Powered by Chemical Reactions?
What are chemical reactions?
Rearrange the atoms in molecules, as in 2H2 + O2 2H2O + energy. This reaction combines hydrogen and oxygen gas to produce water plus energy.
Reverse the process: 2H2O + energy 2H2 + O2. By adding energy we can dissociate water into hydrogen and oxygen.
The energy factor is often left out of chemical reaction formulas, for convenience.
Slide 6
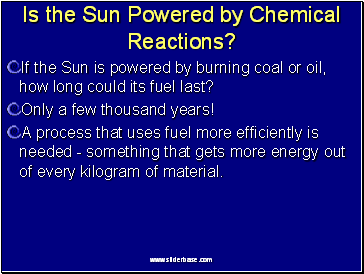
Is the Sun Powered by Chemical Reactions?
If the Sun is powered by burning coal or oil, how long could its fuel last?
Only a few thousand years!
A process that uses fuel more efficiently is needed - something that gets more energy out of every kilogram of material.
Slide 7
Gravity Squeeze?
Gravitational contraction: falling layers of the Sun's material compress the Sun heat energy
drop a book noise! Gravitational potential energy.
A contraction of 40m per day would account for the Sunís energy output.
Efficiency ~ 1/10000 %
Gravity could power the Sun for about 100 million years but the Sun is at least 4 billion years old!
Gravity can't be the Sun's main energy source
But it did help ignite the Sun when it formed
Slide 8
15.2 Mass, Energy, and the Special Theory of Relativity
To understand the way the Sun produces energy, we need to learn a little about nuclear physics and the special theory of relativity.
Contents
- Happy Sun
- Why Does the Sun Shine?
- The Sunís Energy Output
- Gravity Squeeze?
- Elementary Particles (condensed)
- Fusion needs fast moving nuclei
- Fusion Power on Earth
- Why a complicated chain?
- Gravity and Pressure
- Temperature and Pressure
- Hydrostatic Equilibrium
- Other Particles
- Neutrinos
- Neutrino Counting
Last added presentations
- Newtonís Law of Gravity
- Space Radiation
- Practical Applications of Solar Energy
- Gravitation
- Newtonís laws of motion
- Sound
- Upcoming Classes