Intermolecular Forces of AttractionPage
1
1
Slide 1
Intermolecular Forces of Attraction
Slide 2
CA Standards
Students know the atoms and molecules in liquids move in a random pattern relative to one another because the intermolecular forces are too weak to hold the atoms or molecules in a solid form.
Slide 3
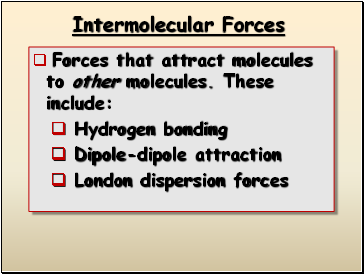
Intermolecular Forces
Forces that attract molecules to other molecules. These include:
Hydrogen bonding
Dipole-dipole attraction
London dispersion forces
Slide 4
Relative Magnitudes of Forces
The types of bonding forces vary in their strength as measured by average bond energy.
Covalent bonds (400 kcal)
Hydrogen bonding (12-16 kcal )
Dipole-dipole interactions (2-0.5 kcal)
London forces (less than 1 kcal)
Strongest
Weakest
Slide 5
Hydrogen Bonding
Bonding between hydrogen and more electronegative neighboring atoms such as oxygen and nitrogen
Base pairing in DNA by hydrogen bonding
Slide 6
Hydrogen Bonding in Water
Slide 7
Polarity
A molecule, such as HF, that has a center of positive charge and a center of negative charge is said to be polar, or to have a dipole moment.
H
F
+
-
Slide 8
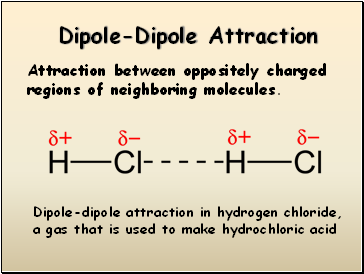
Dipole-Dipole Attraction
Attraction between oppositely charged regions of neighboring molecules.
Dipole-dipole attraction in hydrogen chloride, a gas that is used to make hydrochloric acid
Slide 9
London (Dispersion) Forces
The weakest of intermolecular forces, these forces are proportional to the mass of the molecule
These are the only forces of attraction between completely nonpolar molecules
Large nonpolar molecules may have substantial dispersion forces, resulting in relatively high boiling points
Small nonpolar molecules have weak dispersion forces and exist almost exclusively as gases
Slide 10
London Forces in Hydrocarbons
Contents
- Intermolecular Forces of Attraction
- Intermolecular Forces
- Relative Magnitudes of Forces
- Hydrogen Bonding
- Hydrogen Bonding in Water
- Polarity
- Dipole-Dipole Attraction
- London (Dispersion) Forces
- London Forces in Hydrocarbons
Last added presentations
- Newton’s law of universal gravitation
- Gravitation
- Heat-Energy on the Move
- Newton’s Law of Gravity
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Simulation at NASA for the Space Radiation Effort
- Friction