Galaxies Like the Milky WayPage
1
1
Slide 1
Hydrogen in the Milky Way
Hydrogen is the most abundant element in our galaxy.
The 21-cm Line
Slide 2
Electronic Energy Levels
Atoms emit a photon of light at a specific
wavelength when an electron falls to a lower
energy level.
The 21-cm Line
Slide 3
The resulting dark line spectrum identifies hydrogen.
Hydrogen is detected using a technique called spectroscopy.
The 21-cm Line
Slide 4
An Elementís Signature
An elementís spectrum acts as is its signature.
Each line will always appear in the same location.
The 21-cm Line
Slide 5
Hydrogen only has only one electron spinning around its nucleus.
How can just a spinning electron have energy levels?
The 21-cm Line
Slide 6
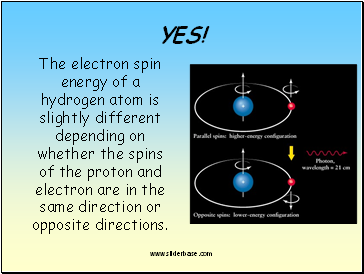
The electron spin energy of a hydrogen atom is slightly different depending on whether the spins of the proton and electron are in the same direction or opposite directions.
YES!
The 21-cm Line
Slide 7
The 21-cm Line
If the spin of the electron changes from the
higher energy level to the lower energy one,
a photon with a wavelength of 21 cm is emitted.
The 21-cm Line
Animation from Nick Strobelís website www.astronomynotes.com
Slide 8
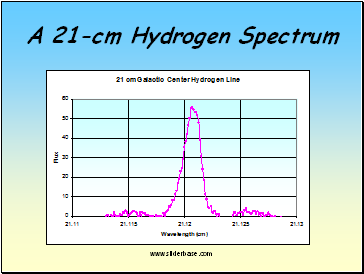
A 21-cm Hydrogen Spectrum
The 21-cm Line
Slide 9
Spectral Lines and Motion
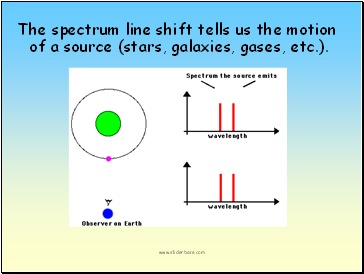
Spectral lines can tell us whether the source is stationary or moving.
If the source is moving, the spectral line will be shifted (called a Doppler shift).
Source at rest
Source moving away
Source moving closer
The 21-cm Line
Slide 10
10
The spectrum line shift tells us the motion of a source (stars, galaxies, gases, etc.).
The 21-cm Line
Slide 11
Shift in wavelength
Stationary
Moving away observer (red shift)
Moving toward observer
(blue shift)
If the source is moving toward the observer, the spectral line will be shifted to a shorter wavelength.
1 2
Contents
- Hydrogen in the Milky Way
- Electronic Energy Levels
- An Elementís Signature
- The 21-cm Line
- A 21-cm Hydrogen Spectrum
- Spectral Lines and Motion
- Shift in wavelength
- Wavelength and velocity
Last added presentations
- Motion
- Newton's Laws
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Radiation Safety and Operations
- Mechanics Lecture
- Newton's laws of motion