The pH ScalePage
1
1
Slide 1
LecturePLUS Timberlake
1
Chapter 9 Acids and Bases
Ionization of Water
The pH Scale
Slide 2
Ionization of Water
LecturePLUS Timberlake
2
Occasionally, in water, a H+ is transferred between H2O molecules
. . . . . . . .
H:O: + :O:H H:O:H + + :O:H-
. . . . . . . .
H H H
water molecules hydronium hydroxide ion (+) ion (-)
Slide 3
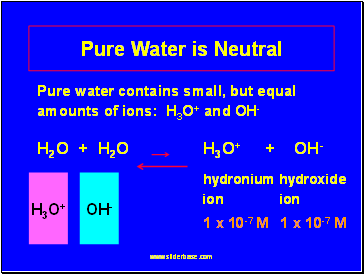
Pure Water is Neutral
LecturePLUS Timberlake
3
Pure water contains small, but equal amounts of ions: H3O+ and OH-
H2O + H2O H3O+ + OH-
hydronium hydroxide ion ion
1 x 10-7 M 1 x 10-7 M
H3O+
OH-
Slide 4
Ion Product of Water Kw
LecturePLUS Timberlake
4
[ ] = Molar concentration
Kw = [ H3O+ ] [ OH- ]
= [ 1 x 10-7 ][ 1 x 10-7 ]
= 1 x 10-14
Slide 5
Acids
LecturePLUS Timberlake
5
Increase H+
HCl (g) + H2O (l) H3O+ (aq) + Cl- (aq)
More [H3O+] than water > 1 x 10-7M
As H3O+ increases, OH- decreases
[H3O+] > [OH-]
H3O+
OH-
Slide 6
Bases
LecturePLUS Timberlake
6
Increase the hydroxide ions (OH-)
H2O
NaOH (s) Na+(aq) + OH- (aq)
More [OH-] than water, [OH-] > 1 x 10-7M
When OH- increases, H3O+ decreases
[OH] > [H3O+]
H3O+
OH-
Slide 7
LecturePLUS Timberlake
7
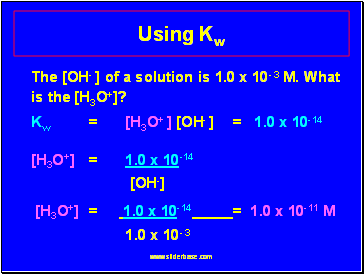
Using Kw
The [OH- ] of a solution is 1.0 x 10- 3 M. What is the [H3O+]?
Kw = [H3O+ ] [OH- ] = 1.0 x 10-14
[H3O+] = 1.0 x 10-14
[OH-]
[H3O+] = 1.0 x 10-14 = 1.0 x 10-11 M
1.0 x 10- 3
Slide 8
LecturePLUS Timberlake
8
Learning Check pH1
The [H3O+] of lemon juice is 1.0 x 10-3 M. What is the [OH-] of the solution?
1) 1.0 x 103 M
2) 1.0 x 10-11 M
3) 1.0 x 1011 M
Slide 9
LecturePLUS Timberlake
9
Solution pH1
The [H3O+] of lemon juice is 1.0 x 10- 3 M. What is the [OH-]?
[OH- ] = 1.0 x 10 -14 = 1.0 x 10-11 M
1.0 x 10 - 3
Slide 10
Using the Calculator
LecturePLUS Timberlake
10
1.0 x 10 -14
Contents
- Ionization of Water
- Pure Water is Neutral
- Ion Product of Water Kw
- Acids
- Bases
- Using the Calculator
- Acid Rain
- Sources of Acid Rain
- Effects of Acid Rain
Last added presentations
- Newton’s third law of motion
- Sound
- Radioactivity and Nuclear Reactions
- Newton’s Laws of Motion
- Soil and Plant Nutrition
- Motion
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal