Quantum Theory of the AtomPage
1
1
Slide 1
Quantum Theory of the Atom
7.1 The Wave Nature of Light
7.2 Quantum Effects and Photons
7.3 The Bohr Theory of the Hydrogen Atom
Slide 2
The Wave Nature of Light
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–2
A wave is a continuously repeating change or oscillation in matter or in a physical field. Light is also a wave.
It consists of oscillations in electric and magnetic fields that travel through space.Visible light, X rays, and radio waves are all forms of electromagnetic radiation.
Slide 3
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–3
The Wave Nature of Light
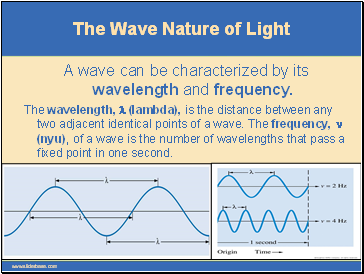
A wave can be characterized by its wavelength and frequency.
The wavelength, l (lambda), is the distance between any two adjacent identical points of a wave. The frequency, n (nyu), of a wave is the number of wavelengths that pass a fixed point in one second.
Slide 4
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–4
So, given the frequency of light, its wavelength can be calculated, or vice versa.
The Wave Nature of Light
The product of the frequency, n (waves/sec) and the wavelength, l (m/wave) would give the speed of the wave in m/s.
Slide 5
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–5
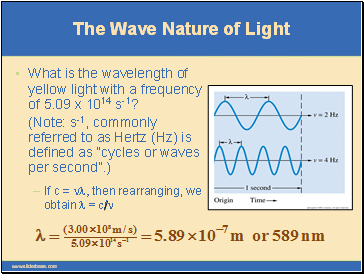
If c = nl, then rearranging, we obtain l = c/n
The Wave Nature of Light
What is the wavelength of yellow light with a frequency of 5.09 x 1014 s-1?
(Note: s-1, commonly referred to as Hertz (Hz) is defined as “cycles or waves per second”.)
Slide 6
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–6
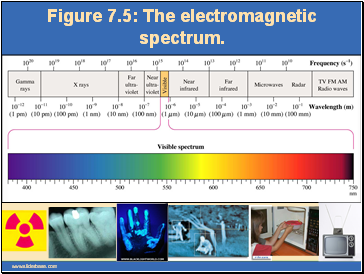
Figure 7.5: The electromagnetic spectrum.
Slide 7
.Copyright © Houghton Mifflin Company.All rights reserved.
Presentation of Lecture Outlines, 7–7
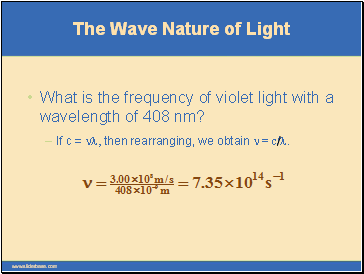
If c = nl, then rearranging, we obtain n = c/l.
What is the frequency of violet light with a wavelength of 408 nm?
The Wave Nature of Light
Slide 8
Copyright © Houghton Mifflin Company.All rights reserved.
Seeing the Light-A New Model of the Atom
Contents
- The Wave Nature of Light
- What is Quantized?
- Quantum Effects and Photons
- Radio Wave Energy
- A Problem to Consider
Last added presentations
- Solar Thermal Energy
- Resource Acquisition and Transport in Vascular Plants
- Practical Applications of Solar Energy
- Radiation Safety and Operations
- Motion
- Radioactivity and Nuclear Reactions
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal