Hard-Soft Acid-Base TheoryPage
1
1
Slide 1
Hard-Soft Acid-Base Theory
Slide 2
Definitions
Arrhenius acids form hydronium ions in water, and bases form hydroxide ions. This definition assumes that water is the solvent.
Brønsted and Lowry expanded upon the Arrhenius definitions, and defined acids as proton donors and bases as proton acceptors. They also introduced the concept of conjugate acid-base pairs.
Slide 3
Other Solvents
For any solvent that can dissociate into a cation and an anion, the cation is the acid, and the anion is the base. Any solute that causes an increase in the concentration of the cation is an acid, those that increase the concentration of the anion are bases.
Slide 4
Lewis Acids & Bases
The Lewis definition further expands the definitions. A base is an electron-pair donor, and an acid is an electron-pair acceptor. The two combine to form an adduct.
A + :B A-B
Slide 5
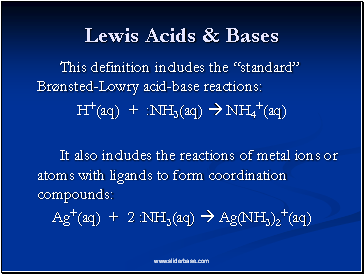
Lewis Acids & Bases
This definition includes the “standard” Brønsted-Lowry acid-base reactions:
H+(aq) + :NH3(aq) NH4+(aq)
It also includes the reactions of metal ions or atoms with ligands to form coordination compounds:
Ag+(aq) + 2 :NH3(aq) Ag(NH3)2+(aq)
Slide 6
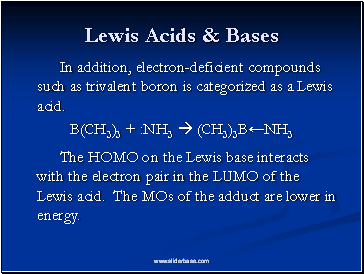
Lewis Acids & Bases
In addition, electron-deficient compounds such as trivalent boron is categorized as a Lewis acid.
B(CH3)3 + :NH3 (CH3)3B←NH3
The HOMO on the Lewis base interacts with the electron pair in the LUMO of the Lewis acid. The MOs of the adduct are lower in energy.
Slide 7
Lewis Acids & Bases
The LUMO and HOMO are called frontier orbitals. If there is a net lowering of energy, the adduct is stable.
Slide 8
BF3 + NH3
The LUMO of the acid, the HOMO of the base and the adduct are shown below:
Slide 9
Lewis Acids & Bases
There is the possibility of competing reaction pathways depending upon which reactants are present, and the relative energies of possible products. As a result, a compound such as water may serve as an acid, a base, an oxidizing agent (with Group IA and IIA metals) or a reducing agent (with F2).
Contents
- Definitions
- Other Solvents
- Lewis Acids & Bases
- Hard and Soft Acids and Bases
- Solubility of Lithium Halides
- Example: Thiocyanate Bonding
- Charge Density – Hard Acids
- Acids
- Bases
- Effect of Linkage Site
- Acid or Base Strength
- Applications of Hard/Soft Theory
- Evidence in Nature
Last added presentations
- Thermal Energy
- Magnetic field uses sound waves to ignite sun's ring of fire
- Ch 9 Nuclear Radiation
- Mechanics Lecture
- Radioactivity and Nuclear Reactions
- Gravitation
- Sensory and Motor Mechanisms