Bohr Diagrams of IonsPage
1
1
Slide 1
Bohr Diagrams of Ions
Lesson 3
August 27th, 2010
Slide 2
Positive and Negative Ions
Noble gases do not form compounds because they have 8 electrons in their outer orbit (shell). This electron arrangement makes them very stable and so they do not react.
Slide 3
When elements form compounds, changes occur in the arrangement of electrons in the outer orbit. – Electrons are gained or lost so that element can have a stable electron arrangement of the closest noble gas. (In other words it will completely fill their outer shell with electrons)
Slide 4
In order for a compound to be stable, it must have a completely filled outer electron shell– aka (stable octet)
Slide 5
Arrangement of outer shell electrons of metals and non-metals
Slide 6
Metals
Tend to have 1, 2, or 3 electrons in the outer orbits (shells)
They lose electrons when they combine with other elements to form positive ions (cations) : note the t in the word think +
They lose electrons, thus they have the same electron arrangement as the Noble gas a row above them
Slide 7
Metal Ion
Example
Sodium: Na Na+
N 12
P 11
Slide 8
Non-Metals
Non-metals – Tend to have 4, 5, 6, or 7 electrons in their outer orbits (shells).
They gain electrons to form negative ions (anions)
They gain electrons, thus they have the same electron arrangement as the Noble gas in the same row.
Slide 9
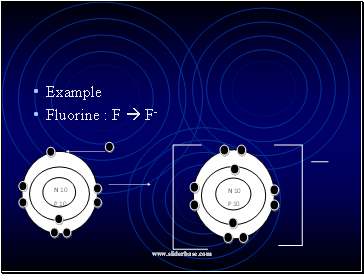
Example
Fluorine : F F-
N 10
P 10
Slide 10
Homework
Draw the ions of the first 20 ions of the periodic table
Contents
Last added presentations
- The Effects of Radiation on Living Things
- Newton's laws of motion
- Gravitation
- Static and Kinetic Friction
- Heat-Energy on the Move
- Solar Thermal Energy
- Sensory and Motor Mechanisms