Atomic Structure IPage
1
1
Slide 1
Atomic Structure
It’s not about Dalton anymore…
Slide 2
First…
To understand the electronic structure of the atom we need to review the properties of electromagnetic radiation.
Slide 3
Figure 7.1
Frequency and Wavelength
c = l n
l = wavelength
n = frequency
C = speed of light
The Wave Nature
of Light
Slide 4
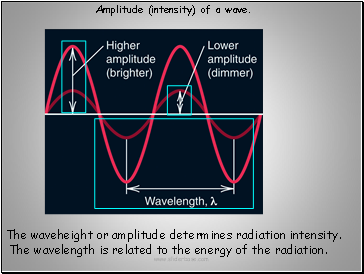
The waveheight or amplitude determines radiation intensity.
The wavelength is related to the energy of the radiation.
Slide 5
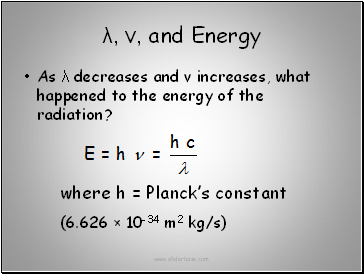
λ, ν, and Energy
As λ decreases and ν increases, what happened to the energy of the radiation?
where h = Planck’s constant
(6.626 × 10-34 m2 kg/s)
Slide 6
Regions of the electromagnetic spectrum.
The infinite number of wavelengths of
electromagnetic radiation have been classified
into groups as shown below.
Slide 7
SOLUTION:
Interconverting Wavelength and Frequency
Use c = ln
= 1.00x10-10 m
= 325x10-2 m
= 473x10-9 m
n =
3x108 m/s
1.00x10-10 m
= 3x1018 s-1
n =
n =
3x108 m/s
325x10-2 m
= 9.23x107 s-1
= 6.34x1014 s-1
Slide 8
SOLUTION:
Calculating the Energy of Radiation from Its Wavelength
After converting cm to m, we can use the energy equation, E = hn combined with n = c/l to find the energy.
E = hc/l
E =
6.626X10-34J*s
3x108m/s
1.20cm
x
= 1.66x10-23J
Slide 9
Particle or Wave?
Different behaviors of waves and particles.
Slide 10
The diffraction pattern caused by light
passing through two adjacent slits.
Slide 11
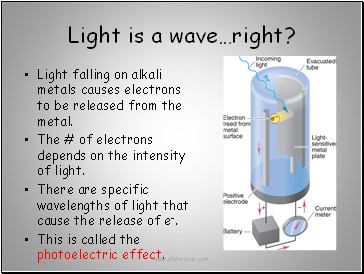
Light is a wave…right?
Light falling on alkali metals causes electrons to be released from the metal.
The # of electrons depends on the intensity of light.
There are specific wavelengths of light that cause the release of e-.
This is called the photoelectric effect.
Slide 12
Contents
- Atomic Structure
- Particle or Wave?
- Light is a wave…right?
- Electrons are particles…right?
- Back to atomic structure…
- Electron locations
- More on electrons as waves
Last added presentations
- Ch 9 Nuclear Radiation
- Practical Applications of Solar Energy
- Madame Marie Curie
- Newton’s Laws of Motion
- Gravitation
- Motion
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal