Acids, Bases & SaltsPage
1
1
Slide 1

Acids, Bases & Salts
Slide 2
TERMS
Topic 10: ACIDS, BASES & SALTS
2
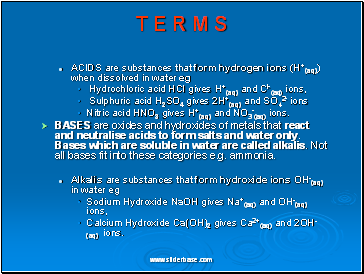
ACIDS are substances that form hydrogen ions (H+(aq)) when dissolved in water eg
Hydrochloric acid HCl gives H+(aq) and Cl-(aq) ions,
Sulphuric acid H2SO4 gives 2H+(aq) and SO42- ions
Nitric acid HNO3 gives H+(aq) and NO3-(aq) ions.
BASES are oxides and hydroxides of metals that react and neutralise acids to form salts and water only. Bases which are soluble in water are called alkalis. Not all bases fit into these categories e.g. ammonia.
Alkalis are substances that form hydroxide ions OH-(aq) in water eg
Sodium Hydroxide NaOH gives Na+(aq) and OH-(aq) ions,
Calcium Hydroxide Ca(OH)2 gives Ca2+(aq) and 2OH-(aq) ions.
جمعرات، 28 ربیع الثانی، 1438
Slide 3

Topic 10: ACIDS, BASES & SALTS
3
In acid solutions there are more H+ ions than OH- ions.
In alkaline solution there are more OH- ions than H+ ions.
Acids that dissociate (ionize) to a large extent are strong electrolytes and Strong Acids.
Acids that dissociate only to a small extent are Weak Acids and weak electrolytes
Bases can be strong or weak depending on the extent to which they dissociate and produce OH– ions in solution. Most metal hydroxides are strong electrolytes and Strong Bases. Ammonia, NH3, is a weak electrolyte and Weak Base.
جمعرات، 28 ربیع الثانی، 1438
Slide 4
Basicity of Acid
Topic 10: ACIDS, BASES & SALTS
4
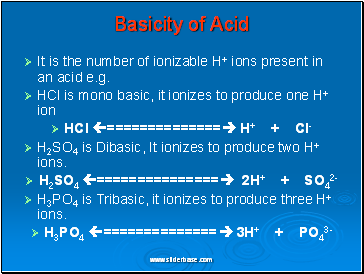
It is the number of ionizable H+ ions present in an acid e.g.
HCl is mono basic, it ionizes to produce one H+ ion
HCl ============== H+ + Cl-
H2SO4 is Dibasic, It ionizes to produce two H+ ions.
H2SO4 =============== 2H+ + SO42-
H3PO4 is Tribasic, it ionizes to produce three H+ ions.
H3PO4 ============== 3H+ + PO43-
جمعرات، 28 ربیع الثانی، 1438
Slide 5

Acidity of a Base
Topic 10: ACIDS, BASES & SALTS
5
It is the ionizable OH- ions present in an alkali. e.g.
NaOH is monoacidic
NaOH ========== Na+ + OH-
Ca(OH)2 is diacidic
Ca(OH)2 ============== Ca2+ + 2OH-
Contents
- TERMS
- Basicity of Acid
- Acidity of a Base
- Common Strong Acids & their Anions
- Common Weak Acids & their Anions
- Naming of Acids
- Formula Writing of Acids
- Properties of Bases
- Naming of Bases
- Formula Writing of Bases
- Physical Properties of Acids & Bases
- Chemical Properties of Acids
- Neutralization
- USES OF ACIDS
- Chemical Properties of Bases
- TYPES OF OXIDES
- SALTS
- Methods of making Soluble Salts
- Making Insoluble Salts
- Types of Salts
- HYDRATED & ANHYDROUS SALTS
- Self Ionization of Water
- pH Graph
- IONIC EQUATIONS
Last added presentations
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Radiation Safety and Operations
- Static and Kinetic Friction
- Madame Marie Curie
- Resource Acquisition and Transport in Vascular Plants
- History of Modern Astronomy
- Newton’s Laws of Motion
