Acids and Bases. ArrheniusPage
1
1
Slide 1
Acids and Bases
Arrhenius
Bronsted-Lowry
Lewis
Slide 2
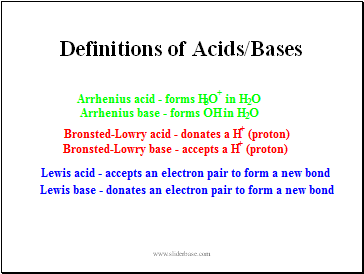
Definitions of Acids/Bases
Slide 3

Measuring Acid Strength Ka
Slide 4
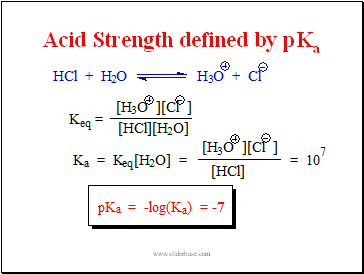
Acid Strength defined by pKa
Slide 5
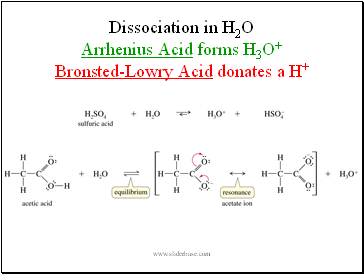
Dissociation in H2O Arrhenius Acid forms H3O+ Bronsted-Lowry Acid donates a H+
Slide 6

Resonance Stabilization
Slide 7
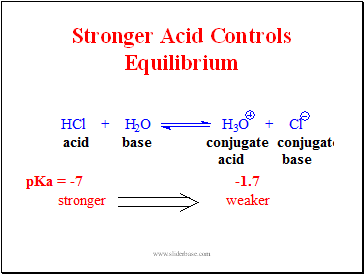
Stronger Acid Controls Equilibrium
Slide 8

Reaction Described with Arrows HCl donates a proton H2O accepts a proton
Slide 9
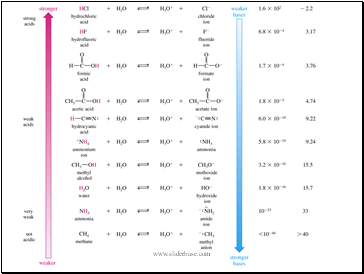
Slide 10

Equilibrium Reactions
Slide 11
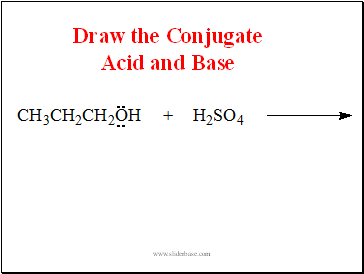
Draw the Conjugate Acid and Base
Slide 12

Propanol is a Base
Slide 13
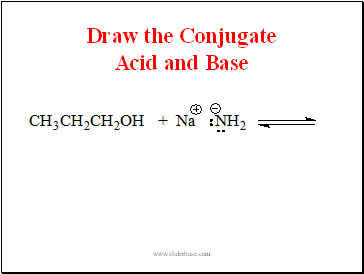
Draw the Conjugate Acid and Base
Slide 14

Propanol is an Acid
Slide 15

Some Acid-Base Reactions
Slide 16

Slide 17

The Effect of Resonance on pKa
Slide 18
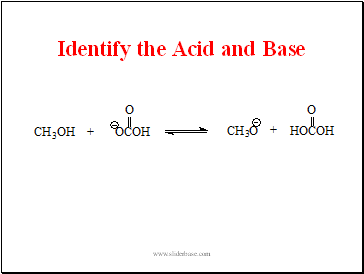
Identify the Acid and Base
Slide 19
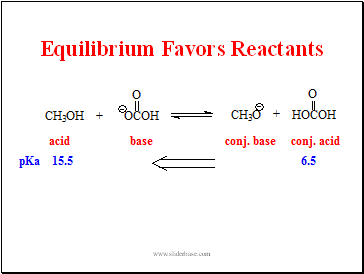
Equilibrium Favors Reactants
Slide 20
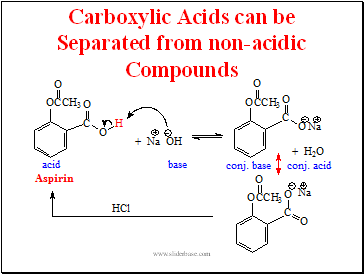
Carboxylic Acids can be Separated from non-acidic Compounds
Slide 21
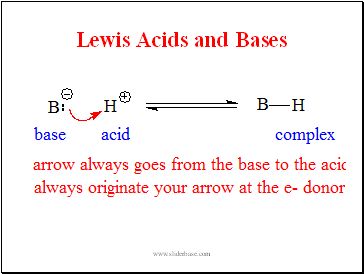
Lewis Acids and Bases
Slide 22

Formation of a new bond
Go to page:
1 2
1 2
Contents
- Definitions of Acids/Bases
- Resonance Stabilization
- Stronger Acid Controls Equilibrium
- Reaction Described with Arrows HCl donates a proton H2O accepts a proton
- Equilibrium Reactions
- Draw the Conjugate Acid and Base
- Propanol is a Base
- Draw the Conjugate Acid and Base
- Propanol is an Acid
- Some Acid-Base Reactions
- The Effect of Resonance on pKa
- Identify the Acid and Base
- Equilibrium Favors Reactants
- Lewis Acids and Bases
- Formation of a new bond
- Draw the Acid-Base Complexes
- Draw Arrows
- Identify the Conjugate Base (i.e. Which H is most acidic?)
Last added presentations
- Newton's laws of motion
- Soil and Plant Nutrition
- Magnetic field uses sound waves to ignite sun's ring of fire
- Ch 9 Nuclear Radiation
- Mechanical, Electromagnetic, Electrical, Chemical and Thermal
- Space Radiation
- Radiation
© 2010-2025 powerpoint presentations
