Matter and the particle modelPage
1
1
Slide 1
What On earth is the matter?
Slide 2
A.
B.
C. ?????
Slide 3
What
in the world is matter?
Slide 4
Matter is…
A. Anything that moves
B. Anything that takes
up space.
C. Something that cries.
Slide 5
B.
Anything that
takes up space!
Slide 6
The
types
Of matter are…
Slide 7
Solids
Liquids
Gases
Slide 8
How does matter change?
Put on your
thinking cap!
Slide 9
solid
liquid
gas
Changes of state
Slide 10
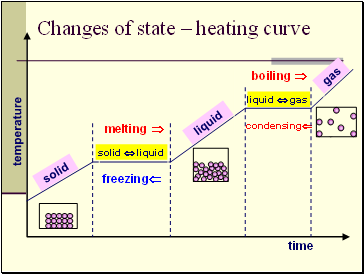
temperature
time
Changes of state – heating curve
Slide 11
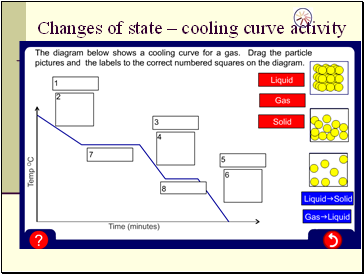
Changes of state – cooling curve activity
Slide 12
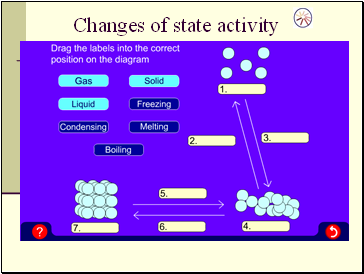
Changes of state activity
Slide 13
The particle model
All substances are made up of particles (atoms, ions or molecules).
These particles are attracted to each other, some strongly and others weakly.
These particles move around (i.e. have kinetic energy).
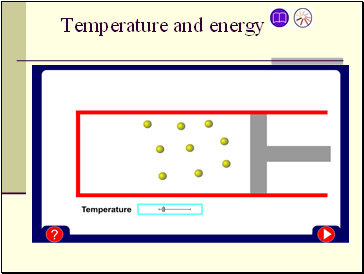
The kinetic energy of particles increases with temperature.
TIME FOR A SONG
Slide 14
Temperature and energy
Slide 15
What is evaporation?
Evaporation occurs when the particles in a liquid escape to form a vapour.
Evaporation can occur at any temperature but it occurs most rapidly at a liquid’s boiling point.
The particles that escape take some energy from the remaining particles and so the temperature of the liquid falls.
Contents
- What On earth is the matter?
- Changes of state activity
- The particle model
- Temperature and energy
- What is evaporation?
Last added presentations
- Newton’s Law of Gravity
- Geophysical Concepts, Applications and Limitations
- Solar Thermal Energy
- History of Modern Astronomy
- Resource Acquisition and Transport in Vascular Plants
- Newton's laws of motion
- Practical Applications of Solar Energy