Acids and alkalisPage
1
1
Slide 1
Acids and Alkalis
Learning Objectives
To know that solutions can be sorted by whether they are: acid, alkali or neutral.
To understand that an alkali reacts with an acid to cancel it out.
To know that indicators show you how acidic or alkaline a solution is.
Slide 2
Acids and alkalis
Solutions can be sorted by whether they are: acid, alkali or neutral.
When a substance dissolves in water it makes a solution.
Slide 3
When the oxide
of some non-metals
dissolve in water
they make an acid.
Acids have a sour taste.
They are corrosive.
Slide 4
Acids react with metals and carbonates
Metal + Acid Salt + Hydrogen
magnesium + magnesium chloride + hydrochloric acid hydrogen
Acid + Carbonate Salt + Water + Carbon
dioxide
sulphuric acid + copper sulphate + water +
copper carbonate carbon dioxide
Slide 5
Acids
Lemon juice contains citric acid, and vinegar contains ethanoic acid.
Some strong acids are hydrochloric acid, sulphuric acid and nitric acid.
Some weak acids are ethanoic acid, citric acid and carbonic acid.
There are many acids present in our everyday lives.
Slide 6
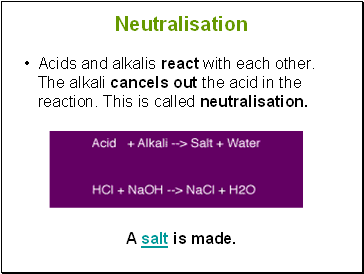
Neutralisation
Acids and alkalis react with each other. The alkali cancels out the acid in the reaction. This is called neutralisation.
A salt is made.
Slide 7
Salts
The salt made depends on the acid and alkali used.
The salt contains the metal atom from the alkali, and part of the acid molecule.
The salts of sulphuric acid are known as sulphates.
The salts of hydrochloric acid are known as chlorides.
The salts of nitric acid are known as nitrates.
Slide 8
Alkalis
When the oxides of some metals dissolve in water they make an alkali solution.
Alkalis react with acids and neutralise them.
Many everyday substances are alkalis.
They feel soapy.
They are corrosive.
Slide 9
Alkalis
Alkalis are present in many cleaning substances in use in our homes.
Kitchen cleaners are alkaline because they contain ammonia or sodium hydroxide, which attack grease.
Calcium hydroxide and sodium hydroxide are strong alkalis.
1 2
Contents
- Acids and Alkalis
- Acids react with metals and carbonates
- Acids
- Neutralisation
- Salts
- Alkalis
- Indicators
- Litmus Test
- Universal Indicator
- Applications of Neutralisation
Last added presentations
- Heat-Energy on the Move
- Radioactivity and Nuclear Reactions
- Geophysical Concepts, Applications and Limitations
- Radiation Safety and Operations
- Thermal Energy
- Newton’s law of universal gravitation
- Understanding Heat Transfer, Conduction, Convection and Radiation