StoichiometryPage
1
1
Slide 1
STOICHIOMETRY
Slide 2
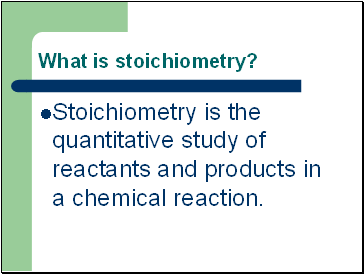
What is stoichiometry?
Stoichiometry is the quantitative study of reactants and products in a chemical reaction.
Slide 3
What You Should Expect
Given : Amount of reactants
Question: how much of products can be formed.
Example
2 A + 2B 3C
Given 20.0 grams of A and sufficient B, how many grams of C can be produced?
Slide 4
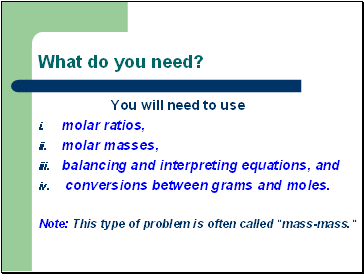
What do you need?
You will need to use
molar ratios,
molar masses,
balancing and interpreting equations, and
conversions between grams and moles.
Note: This type of problem is often called "mass-mass."
Slide 5
Steps Involved in Solving Mass-Mass Stoichiometry Problems
Balance the chemical equation correctly
Using the molar mass of the given substance, convert the mass given to moles.
Construct a molar proportion (two molar ratios set equal to each other)
Using the molar mass of the unknown substance, convert the moles just calculated to mass.
Slide 6
Mole Ratios
A mole ratio converts moles of one compound in a balanced chemical equation into moles of another compound.
Slide 7
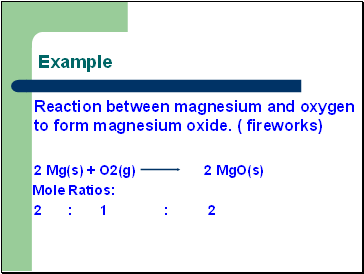
Example
Reaction between magnesium and oxygen to form magnesium oxide. ( fireworks)
2 Mg(s) + O2(g) 2 MgO(s)
Mole Ratios:
2 : 1 : 2
Slide 8
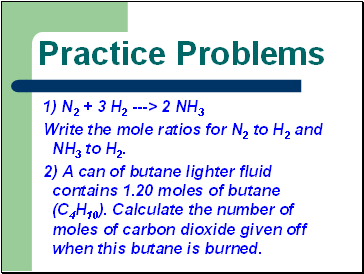
Practice Problems
1) N2 + 3 H2 ---> 2 NH3
Write the mole ratios for N2 to H2 and NH3 to H2.
2) A can of butane lighter fluid contains 1.20 moles of butane (C4H10). Calculate the number of moles of carbon dioxide given off when this butane is burned.
Slide 9
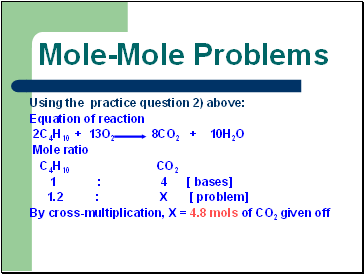
Mole-Mole Problems
Using the practice question 2) above:
Equation of reaction
2C4H10 + 13O2 8CO2 + 10H2O
Mole ratio
C4H10 CO2
1 : 4 [ bases]
1.2 : X [ problem]
By cross-multiplication, X = 4.8 mols of CO2 given off
Slide 10
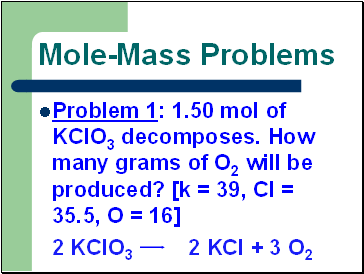
Mole-Mass Problems
Problem 1: 1.50 mol of KClO3 decomposes. How many grams of O2 will be produced? [k = 39, Cl = 35.5, O = 16]
2 KClO3 2 KCl + 3 O2
Slide 11
Contents
- What is stoichiometry?
- Steps Involved in Solving Mass-Mass Stoichiometry Problems
- Mole Ratios
- Practice Problems
- Mole-Mole Problems
- Mole-Mass Problems
- Mass-Mass Problems
- Mass-Volume Problems
- Conversion of mole to volume
- Molar volume
- Practice Problems
Last added presentations
- Solar Thermal Energy
- Practical Applications of Solar Energy
- Understanding Heat Transfer, Conduction, Convection and Radiation
- Geophysical Concepts, Applications and Limitations
- Space Radiation
- Radiation Safety and Operations
- Simulation at NASA for the Space Radiation Effort