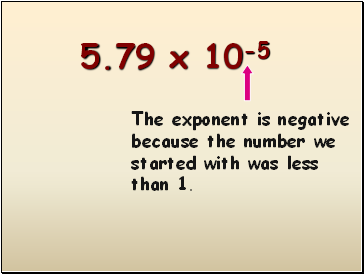Scientific NotationPage
1
1
Slide 1
Scientific Notation
Slide 2
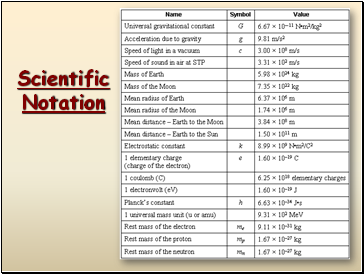
In science, we deal with some very LARGE numbers:
1 mole = 602000000000000000000000
In science, we deal with some very SMALL numbers:
Mass of an electron =
0.000000000000000000000000000000091 kg
Scientific Notation
Slide 3
Imagine the difficulty of calculating the mass of 1 mole of electrons!
0.000000000000000000000000000000091 kg
x 602000000000000000000000
???????????????????????????????????
Slide 4
Scientific Notation:
A method of representing very large or very small numbers in the form:
M x 10n
M is a number between 1 and 10
n is an integer
Slide 5
2 500 000 000
Step #1: Insert an understood decimal point
.
Step #2: Decide where the decimal must end
up so that one number is to its left
Step #3: Count how many places you bounce
the decimal point
1
2
3
4
5
6
7
8
9
Step #4: Re-write in the form M x 10n
Slide 6
2.5 x 109
The exponent is the number of places we moved the decimal.
Slide 7
0.0000579
Step #2: Decide where the decimal must end
up so that one number is to its left
Step #3: Count how many places you bounce
the decimal point
Step #4: Re-write in the form M x 10n
1
2
3
4
5
Slide 8
5.79 x 10-5
The exponent is negative because the number we started with was less than 1.
Slide 9
Performing calculations in scientific notation
ADDITION AND SUBTRACTION
Slide 10
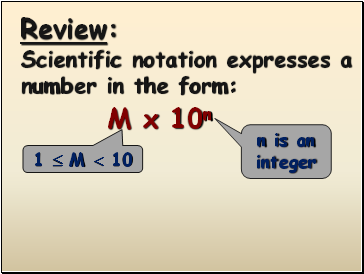
Review:
Scientific notation expresses a number in the form:
M x 10n
1 M 10
n is an integer
Slide 11
4 x 106
+ 3 x 106
IF the exponents are the same, we simply add or subtract the numbers in front and bring the exponent down unchanged.
7
x 106
Slide 12
4 x 106
- 3 x 106
The same holds true for subtraction in scientific notation.
1 2
Contents
Last added presentations
- Geophysical Concepts, Applications and Limitations
- Heat-Energy on the Move
- Newton’s Laws of Motion
- Soil and Plant Nutrition
- Sensory and Motor Mechanisms
- Space Radiation
- Sound